Abstract
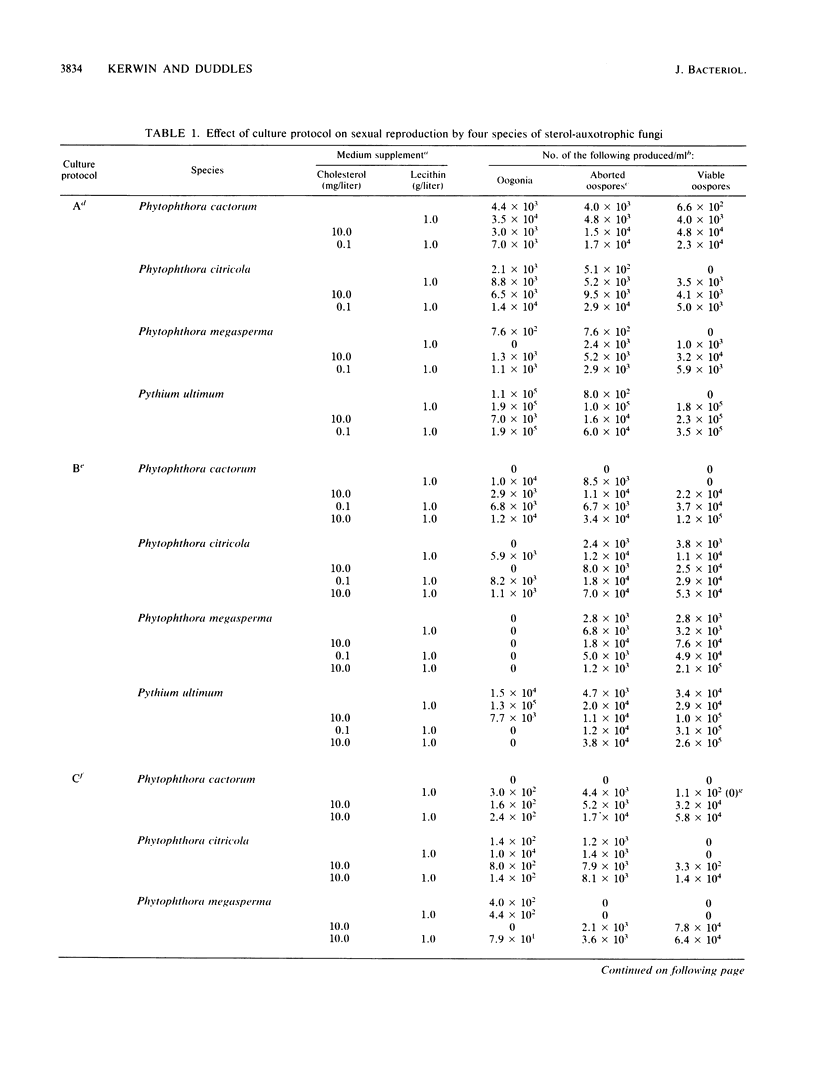
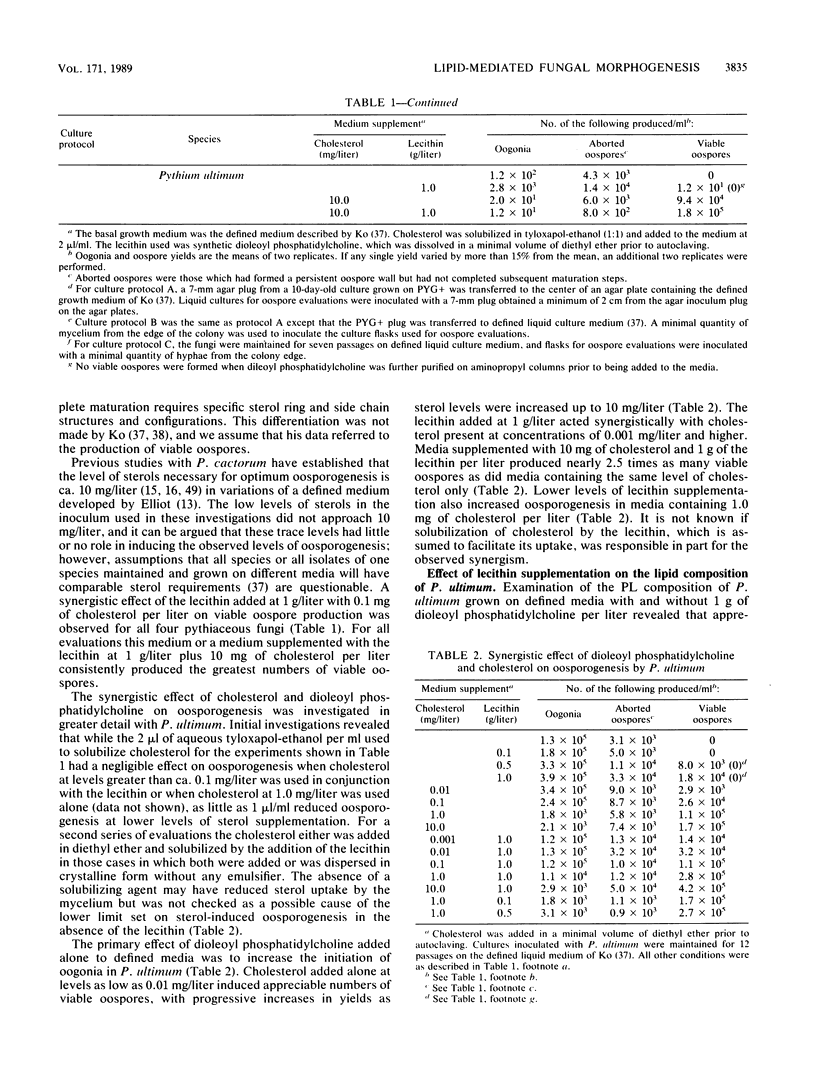
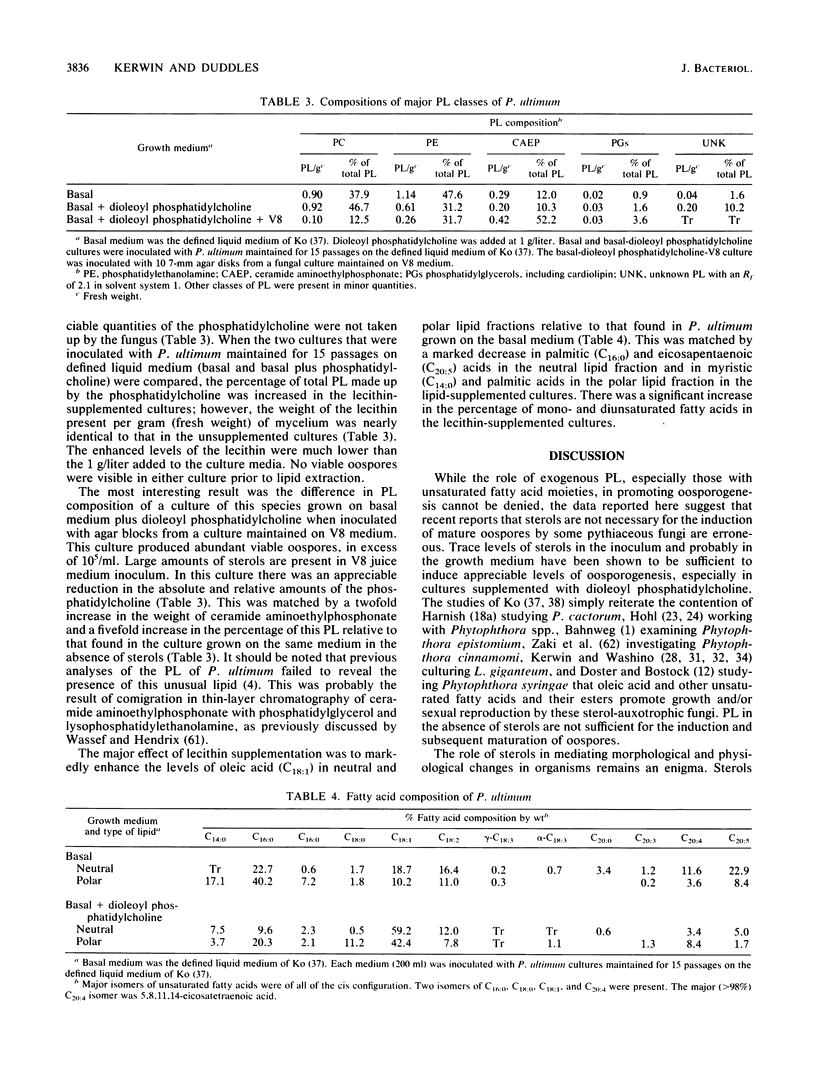
Several genera of oomycete fungi which are incapable of de novo sterol synthesis do not require these compounds for vegetative growth. The requirement for an exogenous source of sterols for sexual reproduction by several members of the Pythiaceae has been questioned by reports of apparent induction and maturation of oospores on defined media supplemented with phospholipids in the absence of sterols. A more detailed examination of this phenomenon suggested that trace levels of sterols in the inoculum of some pythiaceous fungi act synergistically with phospholipid medium supplements containing unsaturated fatty acid moieties to induce oosporogenesis. Phospholipid analysis of one species, Pythium ultimum, suggested that only the fatty acid portion of the exogenous phospholipid is taken up by the fungus. Enrichment of the phospholipid fraction of total cell lipid of P. ultimum with unsaturated fatty acids promoted oospore induction, and enhanced levels of unsaturated fatty acids in the neutral lipid fraction increased oospore viability. For some pythiaceous fungi, the levels of sterols required for the maturation of oospores with appropriate phospholipid medium supplementation suggest that these compounds are necessary only for the sparking and critical domain roles previously described in other fungi.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch K. E. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14(1):47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Bloch K. Sterol structure and membrane function. Curr Top Cell Regul. 1981;18:289–299. doi: 10.1016/b978-0-12-152818-8.50022-0. [DOI] [PubMed] [Google Scholar]
- Bowman R. D., Mumma R. O. The lipids of Phythium ultimum. Biochim Biophys Acta. 1967 Dec 5;144(3):501–510. doi: 10.1016/0005-2760(67)90038-0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Effect of cholesterol on macromolecular synthesis and fatty acid uptake by Mycoplasma capricolum. J Biol Chem. 1981 Jan 10;256(1):87–91. [PubMed] [Google Scholar]
- Dahl J. S., Dahl C. E., Bloch K. Sterols in membranes: growth characteristics and membrane properties of Mycoplasma capricolum cultured on cholesterol and lanosterol. Biochemistry. 1980 Apr 1;19(7):1467–1472. doi: 10.1021/bi00548a032. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Elliott C. G., Sansome E. The influence of sterols on meiosis in Phytophthora cactorum. J Gen Microbiol. 1977 Jan;98(1):141–145. doi: 10.1099/00221287-98-1-141. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gonzales R. A., Parks L. W. Acid-labilization of sterols for extraction from yeast. Biochim Biophys Acta. 1977 Dec 21;489(3):507–509. doi: 10.1016/0005-2760(77)90171-0. [DOI] [PubMed] [Google Scholar]
- HENDRIX J. W. STEROL INDUCTION OF REPRODUCTION AND STIMULATION OF GROWTH OF PYTHIUM AND PHYTOPHTHORA. Science. 1964 May 22;144(3621):1028–1029. doi: 10.1126/science.144.3621.1028. [DOI] [PubMed] [Google Scholar]
- Herman R. P., Herman C. A. Prostaglandins or prostaglandin like substances are implicated in normal growth and development in oomycetes. Prostaglandins. 1985 May;29(5):819–830. doi: 10.1016/0090-6980(85)90140-6. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Yoss N. L. 25-Hydroxysterols increase the permeability of liposomes to Ca2+ and other cations. Biochim Biophys Acta. 1984 Feb 29;770(1):15–21. doi: 10.1016/0005-2736(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Johnston A. M., Aaronson L. R., Martin C. E. The effects of altered levels of phosphatidylcholine and phosphatidylethanolamine on fatty acid desaturase activity and sterol metabolism during temperature acclimation in a choline auxotroph of Neurospora crassa. Biochim Biophys Acta. 1982 Dec 13;713(3):512–518. doi: 10.1016/0005-2760(82)90311-3. [DOI] [PubMed] [Google Scholar]
- Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985 Jan;26(1):135–140. [PubMed] [Google Scholar]
- Kerwin J. L., Simmons C. A., Washino R. K. Eicosanoid regulation of oosporogenesis by Lagenidium giganteum. Prostaglandins Leukot Med. 1986 Aug;23(2-3):173–178. doi: 10.1016/0262-1746(86)90182-4. [DOI] [PubMed] [Google Scholar]
- Kerwin J. L., Washino R. K. Field evaluation of Lagenidium giganteum (Oömycetes: Lagenidiales) and description of a natural epizoötic involving a new isolate of the fungus. J Med Entomol. 1988 Nov;25(6):452–460. doi: 10.1093/jmedent/25.6.452. [DOI] [PubMed] [Google Scholar]
- Kerwin J. L., Washino R. K. Oosporogenesis by Lagenidium giganteum: induction and maturation are regulated by calcium and calmodulin. Can J Microbiol. 1986 Aug;32(8):663–672. doi: 10.1139/m86-123. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Klein I., Moore L., Pastan I. Effect of liposomes containing cholesterol on adenylate cyclase activity of cultured mammalian fibroblasts. Biochim Biophys Acta. 1978 Jan 4;506(1):42–53. doi: 10.1016/0005-2736(78)90433-9. [DOI] [PubMed] [Google Scholar]
- Le Grimellec C., Leblanc G. Effect of membrane cholesterol on potassium transport in Mycoplasma mycoides var. Capri (PG3). Biochim Biophys Acta. 1978 Dec 4;514(1):152–163. doi: 10.1016/0005-2736(78)90085-8. [DOI] [PubMed] [Google Scholar]
- Lorenz R. T., Rodriguez R. J., Lewis T. A., Parks L. W. Characteristics of sterol uptake in Saccharomyces cerevisiae. J Bacteriol. 1986 Sep;167(3):981–985. doi: 10.1128/jb.167.3.981-985.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelhaney R. N., de Gier J., van der Neut-Kok E. C. The effect of alterations in fatty acid composition and cholesterol content on the nonelectrolyte permeability of Acholeplasma laidlawii B cells and derived liposomes. Biochim Biophys Acta. 1973 Mar 16;298(2):500–512. doi: 10.1016/0005-2736(73)90376-3. [DOI] [PubMed] [Google Scholar]
- Mrsny R. J., Volwerk J. J., Griffith O. H. A simplified procedure for lipid phosphorus analysis shows that digestion rates vary with phospholipid structure. Chem Phys Lipids. 1986 Jan;39(1-2):185–191. doi: 10.1016/0009-3084(86)90111-8. [DOI] [PubMed] [Google Scholar]
- Nes W. D., Stafford A. E. Evidence for metabolic and functional discrimination of sterols by Phytophthora cactorum. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3227–3231. doi: 10.1073/pnas.80.11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega A., Mas-Oliva J. Cholesterol effect on enzyme activity of the sarcolemmal (Ca2+ + Mg2+)-ATPase from cardiac muscle. Biochim Biophys Acta. 1984 Jun 27;773(2):231–236. doi: 10.1016/0005-2736(84)90086-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Low C., Bottema C. D., Parks L. W. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim Biophys Acta. 1985 Dec 4;837(3):336–343. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Parks L. W. Structural and physiological features of sterols necessary to satisfy bulk membrane and sparking requirements in yeast sterol auxotrophs. Arch Biochem Biophys. 1983 Sep;225(2):861–871. doi: 10.1016/0003-9861(83)90099-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., Taylor F. R., Parks L. W. A requirement for ergosterol to permit growth of yeast sterol auxotrophs on cholestanol. Biochem Biophys Res Commun. 1982 May 31;106(2):435–441. doi: 10.1016/0006-291x(82)91129-9. [DOI] [PubMed] [Google Scholar]
- Roos D. S., Choppin P. W. Biochemical studies on cell fusion. I. Lipid composition of fusion-resistant cells. J Cell Biol. 1985 Oct;101(4):1578–1590. doi: 10.1083/jcb.101.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Yorek M. A. Membrane lipid composition and cellular function. J Lipid Res. 1985 Sep;26(9):1015–1035. [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Wassef M. K., Hendrix J. W. Ceramide aminoethylphosphonate in the fungus Pythium prolatum. Biochim Biophys Acta. 1976 Jan 18;486(1):172–178. doi: 10.1016/0005-2760(77)90081-9. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., de Greef W. J., van Eyk R. V., Demel R. A., van Deenen L. L. The effect of different fatty acid and sterol composition on the erythritol flux through the cell membrane of Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Mar 16;298(2):479–499. doi: 10.1016/0005-2736(73)90375-1. [DOI] [PubMed] [Google Scholar]


