Abstract
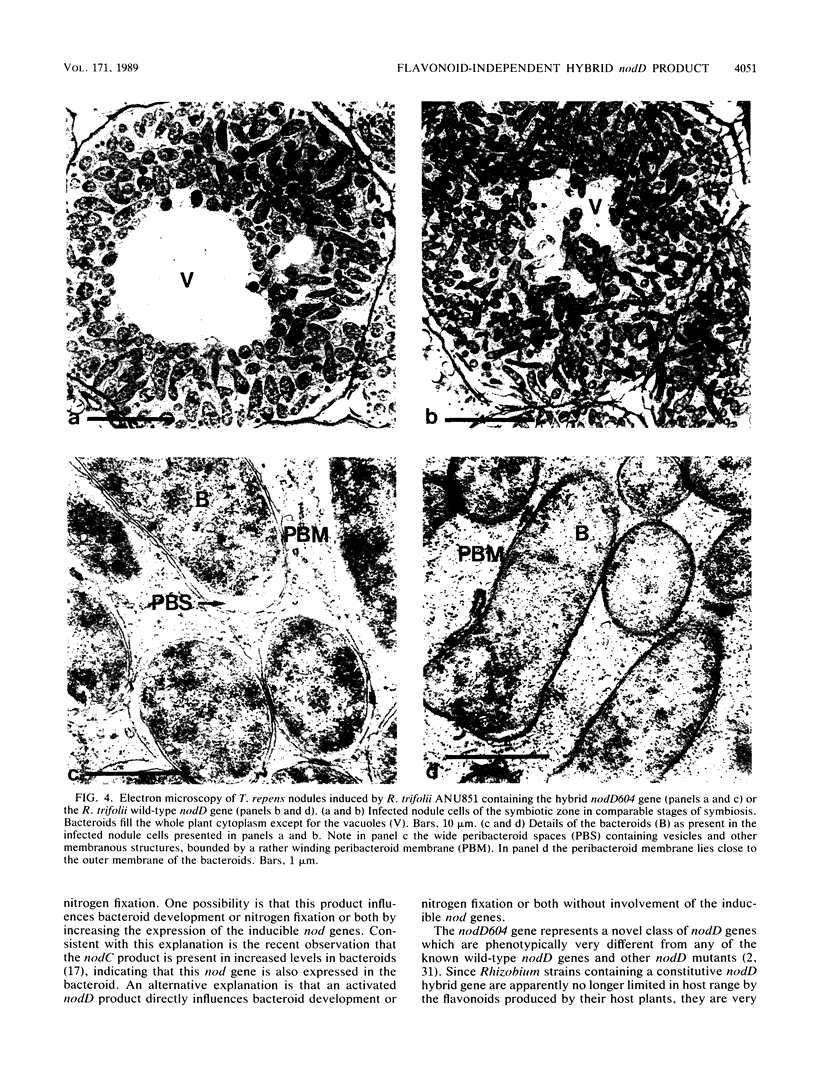
A hybrid nodD gene consisting of 75% of the nodD1 gene of Rhizobium meliloti at the 5' end and 27% of the nodD gene of Rhizobium trifolii at the 3' end activates the six tested inducible nod promoters of Rhizobium leguminosarum, R. trifolii, or R. meliloti to maximal levels, even in the absence of flavonoids. In strains containing such a constitutive activating nodD gene, transcription of nod genes started at the same site as in flavonoid-induced strains containing a wild-type nodD gene. In contrast to heterologous wild-type nodD products, the constitutive activating nodD gene does not cause a limitation of the host range. Furthermore, R. leguminosarum, R. trifolii, and R. meliloti strains containing the constitutive activating nodD gene induce (pseudo) nodules on tropical leguminous plants. Comparison of the symbiotic properties of rhizobia containing the constitutive nodD hybrid gene with those of rhizobia containing various wild-type nodD genes indicates that the activation of the nodD product by flavonoids is of crucial importance during the process of infection thread formation and, surprisingly, also during nitrogen fixation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum E. R., Thompson D. V., Idler K., Chartrain N. Rhizobium japonicum USDA 191 has two nodD genes that differ in primary structure and function. J Bacteriol. 1988 Jan;170(1):12–20. doi: 10.1128/jb.170.1.12-20.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debellé F., Sharma S. B. Nucleotide sequence of Rhizobium meliloti RCR2011 genes involved in host specificity of nodulation. Nucleic Acids Res. 1986 Sep 25;14(18):7453–7472. doi: 10.1093/nar/14.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987 May;6(5):1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff T. T., Fisher R. F., Jacobs T. W., Mulligan J. T., Long S. R. Nucleotide sequence of Rhizobium meliloti 1021 nodulation genes: nodD is read divergently from nodABC. DNA. 1985 Jun;4(3):241–248. doi: 10.1089/dna.1985.4.241. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Brierley H. L., Mulligan J. T., Long S. R. Transcription of Rhizobium meliloti nodulation genes. Identification of a nodD transcription initiation site in vitro and in vivo. J Biol Chem. 1987 May 15;262(14):6849–6855. [PubMed] [Google Scholar]
- Fisher R. F., Swanson J. A., Mulligan J. T., Long S. R. Extended Region of Nodulation Genes in Rhizobium meliloti 1021. II. Nucleotide Sequence, Transcription Start Sites and Protein Products. Genetics. 1987 Oct;117(2):191–201. doi: 10.1093/genetics/117.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttfert M., Horvath B., Kondorosi E., Putnoky P., Rodriguez-Quiñones F., Kondorosi A. At least two nodD genes are necessary for efficient nodulation of alfalfa by Rhizobium meliloti. J Mol Biol. 1986 Oct 5;191(3):411–420. doi: 10.1016/0022-2836(86)90136-1. [DOI] [PubMed] [Google Scholar]
- Honma M. A., Ausubel F. M. Rhizobium meliloti has three functional copies of the nodD symbiotic regulatory gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8558–8562. doi: 10.1073/pnas.84.23.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., Snijdewint F. G., Schilperoort R. A. Identification of the Sym plasmid of Rhizobium leguminosarum strain 1001 and its transfer to and expression in other rhizobia and Agrobacterium tumefaciens. Plasmid. 1982 Jul;8(1):73–82. doi: 10.1016/0147-619x(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Horvath B., Bachem C. W., Schell J., Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987 Apr;6(4):841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Kondorosi E., John M., Schmidt J., Török I., Györgypal Z., Barabas I., Wieneke U., Schell J., Kondorosi A. Organization, structure and symbiotic function of Rhizobium meliloti nodulation genes determining host specificity for alfalfa. Cell. 1986 Aug 1;46(3):335–343. doi: 10.1016/0092-8674(86)90654-9. [DOI] [PubMed] [Google Scholar]
- John M., Schmidt J., Wieneke U., Krüssmann H. D., Schell J. Transmembrane orientation and receptor-like structure of the Rhizobium meliloti common nodulation protein NodC. EMBO J. 1988 Mar;7(3):583–588. doi: 10.1002/j.1460-2075.1988.tb02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Rossen L., Shearman C. A., Johnston A. W., Downie J. A. The nodD gene of Rhizobium leguminosarum is autoregulatory and in the presence of plant exudate induces the nodA,B,C genes. EMBO J. 1985 Dec 16;4(13A):3369–3373. doi: 10.1002/j.1460-2075.1985.tb04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostas K., Kondorosi E., Horvath B., Simoncsits A., Kondorosi A. Conservation of extended promoter regions of nodulation genes in Rhizobium. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1757–1761. doi: 10.1073/pnas.83.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Watson J. M. DNA sequence of Rhizobium trifolii nodulation genes reveals a reiterated and potentially regulatory sequence preceding nodABC and nodFE. Nucleic Acids Res. 1986 Apr 11;14(7):2891–2903. doi: 10.1093/nar/14.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surin B. P., Downie J. A. Characterization of the Rhizobium leguminosarum genes nodLMN involved in efficient host-specific nodulation. Mol Microbiol. 1988 Mar;2(2):173–183. doi: 10.1111/j.1365-2958.1988.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Spaink H. P., van Brussel A. A., Okker R. J., Lugtenberg B. J. Induction of the nodA promoter of Rhizobium leguminosarum Sym plasmid pRL1JI by plant flavanones and flavones. J Bacteriol. 1987 Jan;169(1):198–204. doi: 10.1128/jb.169.1.198-204.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brussel A. A., Costerton J. W., Child J. J. Nitrogen fixation by Rhizobium sp. 32H1. A morphological and ultrastructural comparison of asymbiotic and symbiotic nitrogen-fixing forms. Can J Microbiol. 1979 Mar;25(3):352–361. doi: 10.1139/m79-055. [DOI] [PubMed] [Google Scholar]