Abstract
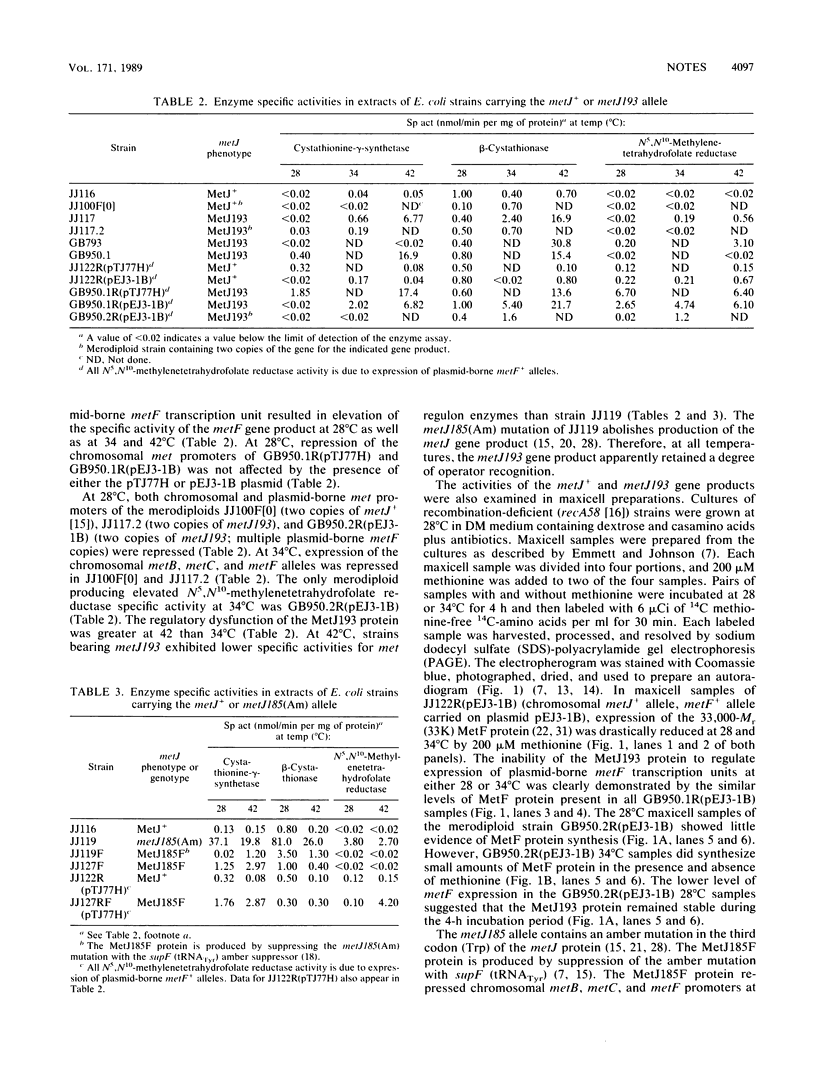
At 28 degrees C, but not at 34 or 42 degrees C, strains with the metJ193 allele repressed chromosomal met genes but not a plasmid-borne met promoter. Increasing the metJ193 gene dosage to two copies resulted in overrepression of chromosomal and plasmid-borne met promoters at 28 degrees C. Suppressing the metJ185 amber mutation with supF (tRNATyr) produced the MetJ185F protein. Strains producing MetJ185F repressed chromosomal met promoters but not a plasmid-borne met promoter at 42 degrees C. These are the first known defective MetJ proteins with documented temperature-dependent function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass S., Sorrells V., Youderian P. Mutant Trp repressors with new DNA-binding specificities. Science. 1988 Oct 14;242(4876):240–245. doi: 10.1126/science.3140377. [DOI] [PubMed] [Google Scholar]
- Belfaiza J., Guillou Y., Margarita D., Perrin D., Saint Girons I. Operator-constitutive mutations of the Escherichia coli metF gene. J Bacteriol. 1987 Feb;169(2):670–674. doi: 10.1128/jb.169.2.670-674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett M. R., Johnson J. R. Control of metF gene expression in maxicell preparations of Escherichia coli K-12: reversible action of the metJ protein and effect of vitamin B12. J Bacteriol. 1986 Dec;168(3):1491–1494. doi: 10.1128/jb.168.3.1491-1494.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Greene R. C., Krueger J. H. Isolation and characterization of specialized lambda transducing bacteriophage carrying the metBJF methionine gene cluster. J Bacteriol. 1977 Sep;131(3):795–800. doi: 10.1128/jb.131.3.795-800.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Liljestrand C. A. Plaque assay for lambda transducing phage carrying the E. coli metB gene. Mol Gen Genet. 1983;190(3):527–530. doi: 10.1007/BF00331087. [DOI] [PubMed] [Google Scholar]
- Kirby T. W., Hindenach B. R., Greene R. C. Regulation of in vivo transcription of the Escherichia coli K-12 metJBLF gene cluster. J Bacteriol. 1986 Mar;165(3):671–677. doi: 10.1128/jb.165.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Liljestrand-Golden C. A., Johnson J. R. Physical organization of the metJB component of the Escherichia coli K-12 metJBLF gene cluster. J Bacteriol. 1984 Feb;157(2):413–419. doi: 10.1128/jb.157.2.413-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence M. C., Rupert C. S. Convenient construction of recA deletion derivatives of Escherichia coli. J Bacteriol. 1983 Oct;156(1):458–459. doi: 10.1128/jb.156.1.458-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Rossi J. J., Landy A. Structure and organization of the two tRNATyr gene clusters on the E. coli chromosome. Cell. 1979 Mar;16(3):523–534. doi: 10.1016/0092-8674(79)90027-8. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Belfaiza J., Guillou Y., Perrin D., Guiso N., Bârzu O., Cohen G. N. Interactions of the Escherichia coli methionine repressor with the metF operator and with its corepressor, S-adenosylmethionine. J Biol Chem. 1986 Aug 15;261(23):10936–10940. [PubMed] [Google Scholar]
- Saint-Girons I., Duchange N., Cohen G. N., Zakin M. M. Structure and autoregulation of the metJ regulatory gene in Escherichia coli. J Biol Chem. 1984 Nov 25;259(22):14282–14285. [PubMed] [Google Scholar]
- Saint-Girons I., Duchange N., Zakin M. M., Park I., Margarita D., Ferrara P., Cohen G. N. Nucleotide sequence of metF, the E. coli structural gene for 5-10 methylene tetrahydrofolate reductase and of its control region. Nucleic Acids Res. 1983 Oct 11;11(19):6723–6732. doi: 10.1093/nar/11.19.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Girons I., Parsot C., Zakin M. M., Bârzu O., Cohen G. N. Methionine biosynthesis in Enterobacteriaceae: biochemical, regulatory, and evolutionary aspects. CRC Crit Rev Biochem. 1988;23 (Suppl 1):S1–42. doi: 10.3109/10409238809083374. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeman R., Coleman T., Redfield B., Greene R. C., Smith A. A., Saint-Girons I., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: effect of metJ gene product and S-adenosylmethionine on the in vitro expression of the metB, metL and metJ genes. Biochem Biophys Res Commun. 1985 Dec 17;133(2):731–739. doi: 10.1016/0006-291x(85)90965-9. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Redfield B., Coleman T., Greene R. C., Smith A. A., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: Effect of metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3601–3605. doi: 10.1073/pnas.82.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. A., Greene R. C. Cloning of the methionine regulatory gene, metJ, of Escherichia coli K12 and identification of its product. J Biol Chem. 1984 Nov 25;259(22):14279–14281. [PubMed] [Google Scholar]
- Smith A. A., Greene R. C., Kirby T. W., Hindenach B. R. Isolation and characterization of the product of the methionine-regulatory gene metJ of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6104–6108. doi: 10.1073/pnas.82.18.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N., Hoess R. The molecular genetics of bacteriophage P1. Annu Rev Genet. 1983;17:123–154. doi: 10.1146/annurev.ge.17.120183.001011. [DOI] [PubMed] [Google Scholar]
- Treat M. L., Weaver M. L., Emmett M. R., Johnson J. R. Mutagenesis of the metJBLF gene cluster with transposon Tn5: localization of the metF transcription unit. Mol Gen Genet. 1984;193(2):370–375. doi: 10.1007/BF00330695. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Plamann L. S., Stauffer G. V. Mutations affecting the regulation of the metB gene of Salmonella typhimurium LT2. J Bacteriol. 1987 Jan;169(1):126–130. doi: 10.1128/jb.169.1.126-130.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Cloning and initial characterization of the metJ and metB genes from Salmonella typhimurium LT2. Gene. 1985;35(1-2):187–197. doi: 10.1016/0378-1119(85)90171-4. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Nucleotide sequence and biochemical characterization of the metJ gene from Salmonella typhimurium LT2. Nucleic Acids Res. 1985 Feb 11;13(3):673–685. doi: 10.1093/nar/13.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. W., Mantsch H. H., Arrondo J. L., Saint-Girons I., Guillou Y., Cohen G. N., Bârzu O. Fourier transform infrared investigation of the Escherichia coli methionine aporepressor. Biochemistry. 1987 May 19;26(10):2706–2711. doi: 10.1021/bi00384a009. [DOI] [PubMed] [Google Scholar]