Abstract
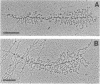
The distinctive double Christmas tree morphology of rRNA operons as visualized by electron microscopy makes them easy to recognize in chromatin spreads from Escherichia coli. On the basis of the pattern of nascent transcripts on nearby transcription units and the relative distances of the operons from one another and the replication origin, we are now able to specifically identify five of the seven rRNA operons in E. coli. The use of rRNA operons as markers of both position and distance has resulted in the morphological mapping of a significant portion of the E. coli chromosome; over 600 kilobase pairs in the 84- to 90-min and 72-min regions can now be recognized. Since individual rRNA operons could be identified, direct comparisons could be made of their transcriptional activities. As judged by the densities of RNA polymerases along the operons, rrnA, rrnB, rrnC, rrnD, and rrnE were all transcribed at similar levels, with one RNA polymerase every 85 base pairs. The ability to recognize individual operons and specific regions of the chromosome allows direct comparisons of various genetic parameters.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C. L., Squires C. Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4922–4926. doi: 10.1073/pnas.76.10.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry G., Squires C., Squires C. L. Attenuation and processing of RNA from the rplJL--rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3331–3335. doi: 10.1073/pnas.77.6.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell D., Davis G., Gosink M., Post L., Nomura M., Kestler H., Zengel J. M., Lindahl L. Nucleotide sequence of the alpha ribosomal protein operon of Escherichia coli. Nucleic Acids Res. 1985 Jun 11;13(11):3891–3903. doi: 10.1093/nar/13.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M., Moelleken A., Maley G. F., Maley F. Purification and properties of T4 phage thymidylate synthetase produced by the cloned gene in an amplification vector. J Biol Chem. 1983 Feb 10;258(3):2045–2051. [PubMed] [Google Scholar]
- Bell A. W., Buckel S. D., Groarke J. M., Hope J. N., Kingsley D. H., Hermodson M. A. The nucleotide sequences of the rbsD, rbsA, and rbsC genes of Escherichia coli K12. J Biol Chem. 1986 Jun 15;261(17):7652–7658. [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Construction of a single-copy promoter vector and its use in analysis of regulation of the transposon Tn10 tetracycline resistance determinant. J Bacteriol. 1984 Jun;158(3):910–919. doi: 10.1128/jb.158.3.910-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. L., Osheim Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988 Jun;2(6):754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Boros I., Csordás-Tóth E., Kiss A., Kiss I., Török I., Udvardy A., Udvardy K., Venetianer P. Identification of two new promoters probably involved in the transcription of a ribosomal RNA gene of Escherichia coli. Biochim Biophys Acta. 1983 Mar 10;739(2):173–180. doi: 10.1016/0167-4781(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Boros I., Kiss A., Venetianer P. Physical map of the seven ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1979;6(5):1817–1830. doi: 10.1093/nar/6.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J. When polymerases collide: replication and the transcriptional organization of the E. coli chromosome. Cell. 1988 Jun 3;53(5):679–686. doi: 10.1016/0092-8674(88)90086-4. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brückner R., Matzura H. In vivo synthesis of a polycistronic messenger RNA for the ribosomal proteins L11, L1, L10 and L7/12 in Escherichia coli. Mol Gen Genet. 1981;183(2):277–282. doi: 10.1007/BF00270629. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Delcuve G., Downing W., Lewis H., Dennis P. P. Nucleotide sequence of the proximal portion of the RNA polymerase beta subunit gene of Escherichia coli. Gene. 1980 Nov;11(3-4):367–373. doi: 10.1016/0378-1119(80)90076-1. [DOI] [PubMed] [Google Scholar]
- Delius H., Westphal H., Axelrod N. Length measurements of RNA synthesized in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1973 Mar 15;74(4):677–687. doi: 10.1016/0022-2836(73)90056-9. [DOI] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger M., Bjornsti M. A., Uetz T., Hobot J. A., Kellenberger E. Intracellular location of the histonelike protein HU in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4757–4768. doi: 10.1128/jb.170.10.4757-4768.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood M., Nomura M. Chromosomal locations of the genes for rRNA in Escherichia coli K-12. J Bacteriol. 1982 Feb;149(2):458–468. doi: 10.1128/jb.149.2.458-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French S., Martin K., Patterson T., Bauerle R., Miller O. L., Jr Electron microscopic visualization of trp operon expression in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4638–4642. doi: 10.1073/pnas.82.14.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980 Jun 15;140(1):57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Yanofsky C. Cloning and characterization of the gene for Escherichia coli tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1981 Dec;148(3):941–949. doi: 10.1128/jb.148.3.941-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamkalo B. A., Miller O. L., Jr Electronmicroscopy of genetic activity. Annu Rev Biochem. 1973;42:379–396. doi: 10.1146/annurev.bi.42.070173.002115. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heller K., Kadner R. J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985 Mar;161(3):904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann S., Miller O. L., Jr Visualization of ribosomal ribonucleic acid synthesis in a ribonuclease III-Deficient strain of Escherichia coli. J Bacteriol. 1977 Nov;132(2):718–722. doi: 10.1128/jb.132.2.718-722.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope J. N., Bell A. W., Hermodson M. A., Groarke J. M. Ribokinase from Escherichia coli K12. Nucleotide sequence and overexpression of the rbsK gene and purification of ribokinase. J Biol Chem. 1986 Jun 15;261(17):7663–7668. [PubMed] [Google Scholar]
- Howard P. K., Shaw J., Otsuka A. J. Nucleotide sequence of the birA gene encoding the biotin operon repressor and biotin holoenzyme synthetase functions of Escherichia coli. Gene. 1985;35(3):321–331. doi: 10.1016/0378-1119(85)90011-3. [DOI] [PubMed] [Google Scholar]
- Hudson L., Rossi J., Landy A. Dual function transcripts specifying tRNA and mRNA. Nature. 1981 Dec 3;294(5840):422–427. doi: 10.1038/294422a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson S., Gourse R. L., Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983 Jul;33(3):865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Kiss A., Sain B., Venetianer P. The number of rRNA genes in Escherichia coli. FEBS Lett. 1977 Jul 1;79(1):77–79. doi: 10.1016/0014-5793(77)80354-2. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laird C. D., Wilkinson L. E., Foe V. E., Chooi W. Y. Analysis of chromatin-associated fiber arrays. Chromosoma. 1976 Oct 28;58(2):169–190. doi: 10.1007/BF00701357. [DOI] [PubMed] [Google Scholar]
- Lee J. S., An G., Friesen J. D., Fill N. P. Location of the tufB promoter of E. coli: cotranscription of tufB with four transfer RNA genes. Cell. 1981 Jul;25(1):251–258. doi: 10.1016/0092-8674(81)90250-6. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Post L., Zengel J., Gilbert S. F., Strycharz W. A., Nomura M. Mapping of ribosomal protein genes by in vitro protein synthesis using DNA fragments of lambdafus3 transducing phage DNA as templates. J Biol Chem. 1977 Oct 25;252(20):7365–7383. [PubMed] [Google Scholar]
- Lindahl S., Yamamoto M., Nomura M. Mapping of a cluster of genes for components of the transcriptional and translational machineries of Escherichia coli. J Mol Biol. 1977 Jan 5;109(1):23–47. doi: 10.1016/s0022-2836(77)80044-2. [DOI] [PubMed] [Google Scholar]
- Lindström P. H., Stüber D., Björk G. R. Genetic organization and transcription from the gene (trmA) responsible for synthesis of tRNA (uracil-5)-methyltransferase by Escherichia coli. J Bacteriol. 1985 Dec;164(3):1117–1123. doi: 10.1128/jb.164.3.1117-1123.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Bustin M., Miller O. L., Jr Electron microscopic analysis of chromosome metabolism in the Drosophila melanogaster embryo. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):741–754. doi: 10.1101/sqb.1978.042.01.075. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Electron microscopic analysis of chromatin replication in the cellular blastoderm Drosophila melanogaster embryo. Cell. 1977 Nov;12(3):795–804. doi: 10.1016/0092-8674(77)90278-1. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Sullivan N. L., Miller O. L., Jr Visualization of the silk fibroin transcription unit and nascent silk fibroin molecules on polyribosomes of Bombyx mori. Prog Nucleic Acid Res Mol Biol. 1976;19:313–318. doi: 10.1016/s0079-6603(08)60928-9. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A. Visualization of RNA synthesis on chromosomes. Int Rev Cytol. 1972;33:1–25. doi: 10.1016/s0074-7696(08)61446-1. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Shibuya M., Kuchino Y., Kaziro Y. Transcription of the E. coli tufB gene: cotranscription with four tRNA genes and inhibition by guanosine-5'-diphosphate-3'-diphosphate. Mol Gen Genet. 1981;183(1):13–19. doi: 10.1007/BF00270131. [DOI] [PubMed] [Google Scholar]
- Morgan E. A., Ikemura T., Nomura M. Identification of spacer tRNA genes in individual ribosomal RNA transcription units of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2710–2714. doi: 10.1073/pnas.74.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J. E., Grant R. A., Ho Y. S., Platt T. Maximizing gene expression from plasmid vectors containing the lambda PL promoter: strategies for overproducing transcription termination factor rho. Proc Natl Acad Sci U S A. 1985 Jan;82(1):88–92. doi: 10.1073/pnas.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Conrad B. Improved programs for DNA and protein sequence analysis on the IBM personal computer and other standard computer systems. Nucleic Acids Res. 1986 Jan 10;14(1):443–454. doi: 10.1093/nar/14.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Morgan E. A. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr, Beyer A. L. Two Drosophila chorion genes terminate transcription in discrete regions near their poly(A) sites. EMBO J. 1986 Dec 20;5(13):3591–3596. doi: 10.1002/j.1460-2075.1986.tb04687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Salomatina I. S., Shuvaeva T. M., Lipkin V. M., Sverdlov E. D. The primary structure of E. coli RNA polymerase, Nucleotide sequence of the rpoC gene and amino acid sequence of the beta'-subunit. Nucleic Acids Res. 1982 Jul 10;10(13):4035–4044. doi: 10.1093/nar/10.13.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Arfsten A. E., Reusser F., Nomura M. DNA sequences of promoter regions for the str and spc ribosomal protein operons in E. coli. Cell. 1978 Sep;15(1):215–229. doi: 10.1016/0092-8674(78)90096-x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Ho Y. S., Shatzman A. The use of pKc30 and its derivatives for controlled expression of genes. Methods Enzymol. 1983;101:123–138. doi: 10.1016/0076-6879(83)01009-5. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J. Localization of the HU protein on the Escherichia coli nucleoid. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):439–447. doi: 10.1101/sqb.1978.042.01.047. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Nedospasov S. A., Bakayev V. V., Bakayeva T. G., Georgiev G. P. Histone-like proteins in the purified Escherichia coli deoxyribonucleoprotein. Nucleic Acids Res. 1977 Aug;4(8):2725–2745. doi: 10.1093/nar/4.8.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. The structure of DNA. Cold Spring Harb Symp Quant Biol. 1953;18:123–131. doi: 10.1101/sqb.1953.018.01.020. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Saraste M., Eberle A. N. DNA sequence around the Escherichia coli unc operon. Completion of the sequence of a 17 kilobase segment containing asnA, oriC, unc, glmS and phoS. Biochem J. 1984 Dec 15;224(3):799–815. doi: 10.1042/bj2240799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K., Nagata A., Kano Y., Imamoto F. Isolation and characterization of nucleoid proteins from Escherichia coli. Mol Gen Genet. 1984;196(2):217–224. doi: 10.1007/BF00328053. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Archer R. H., Lindahl L. The nucleotide sequence of the Escherichia coli fus gene, coding for elongation factor G. Nucleic Acids Res. 1984 Feb 24;12(4):2181–2192. doi: 10.1093/nar/12.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M., Patterson T. A., Court D. L. Analysis of nutR, a site required for transcription antitermination in phage lambda. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4514–4518. doi: 10.1073/pnas.84.13.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Zurawski S. M. Structure of the Escherichia coli S10 ribosomal protein operon. Nucleic Acids Res. 1985 Jun 25;13(12):4521–4526. doi: 10.1093/nar/13.12.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Deen L. T., Smith D. W. Temporal sequence of events during the initiation process in Escherichia coli deoxyribonucleic acid replication: roles of the dnaA and dnaC gene products and ribonucleic acid polymerase. J Bacteriol. 1977 Mar;129(3):1466–1475. doi: 10.1128/jb.129.3.1466-1475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]