Abstract
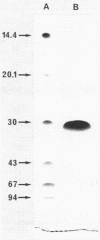
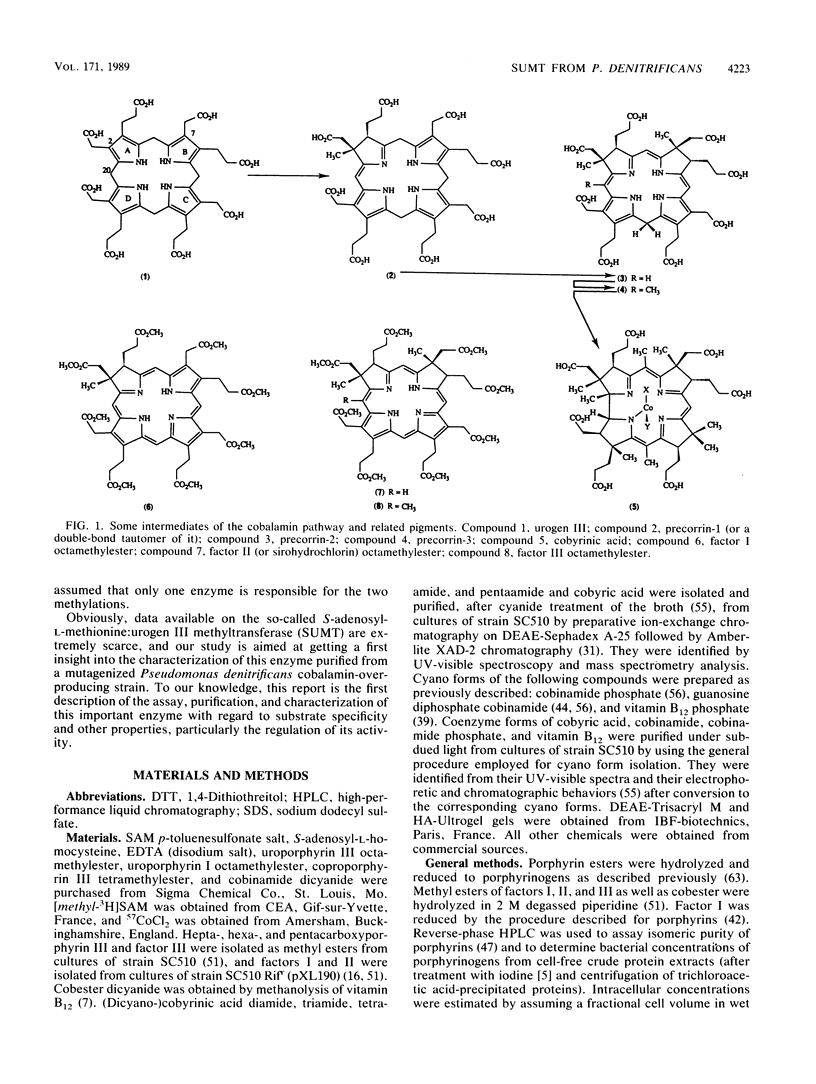
S-Adenosyl-L-methionine:uroporphyrinogen III methyltransferase (SUMT), the enzyme of the cobalamin biosynthetic pathway which catalyzes C methylation of uroporphyrinogen III, was purified about 150-fold to homogeneity from extracts of a recombinant strain of Pseudomonas denitrificans derived from a cobalamin-overproducing strain by ammonium sulfate fractionation, anion-exchange chromatography, and hydroxyapatite chromatography. The purified protein has an isoelectric point of 6.4 and molecular weights of 56,500 as estimated by gel filtration and 30,000 as estimated by gel electrophoresis under denaturing conditions, suggesting that the active enzyme is a homodimer. It does not contain a chromophoric prosthetic group and does not seem to require metal ions or cofactors for activity. SUMT catalyzes the two successive C-2 and C-7 methylation reactions involved in the conversion of uroporphyrinogen III to precorrin-2 via the intermediate formation of precorrin-1. In vitro studies suggest that the intermediate monomethylated product (precorrin-1) is released from the protein and then added back to the enzyme for the second C-methylation reaction. The pH optimum was 7.7, the Km values for S-adenosyl-L-methionine and uroporphyrinogen III were 6.3 and 1.0 microM, respectively, and the turnover number was 38 h-1. The enzyme activity was shown to be completely insensitive to feedback inhibition by cobalamin and corrinoid intermediates tested at physiological concentration. At uroporphyrinogen III concentrations above 2 microM, SUMT exhibited a substrate inhibition phenomenon. It is suggested that this property might play a regulatory role in cobalamin biosynthesis in the cobalamin-overproducing strain studied.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Battersby A. R., Ihara M., McDonald E., Redfern J. R., Golding B. T. Biosynthesis of porphyrins and related macrocycles. Part 9. Biosynthesis of the corrin macrocycle of vitamin B12 including the sterochemistry of C-methylation in ring. J Chem Soc Perkin 1. 1977;2:158–166. doi: 10.1039/p19770000158. [DOI] [PubMed] [Google Scholar]
- Battersby A. R., McDonald E., Hollenstein R., Ihara M., Satoh F., Williams D. C. Biosynthesis of porphyrins and related macrocycles. Part 10. Vitamin B12: biochemical derivation of cobyrinic acid from uroporphyrinogen III. Studies with corresponding ring c methyl heptacarboxylic prophyrinogen, and proof of seven intact methyl transfers. J Chem Soc Perkin 1. 1977;2:166–178. doi: 10.1039/p19770000166. [DOI] [PubMed] [Google Scholar]
- Bergmann K. H., Deeg R., Gneuss K. D., Kriemler H. P., Müller G. Zur Cobyrinäure-Biosynthese. Gewinnung von Zwischenprodukten der Cobyrinsäure-Biosynthese mit Zellsuspensionen von Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1977 Oct;358(10):1315–1323. [PubMed] [Google Scholar]
- Berhauer K., Wagner F., Michna H., Rapp P., Vogelmann H. Zur Chemie und Biochemie der Corrinoide, XXIX. Biogenesewege von der Cobyrinsäure zur Cobysäure und zum Cobinamid bei Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1968 Oct;349(10):1297–1309. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Cameron B., Briggs K., Pridmore S., Brefort G., Crouzet J. Cloning and analysis of genes involved in coenzyme B12 biosynthesis in Pseudomonas denitrificans. J Bacteriol. 1989 Jan;171(1):547–557. doi: 10.1128/jb.171.1.547-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Substrate inhibition. Methods Enzymol. 1979;63:500–513. [PubMed] [Google Scholar]
- Dauner H. O., Müller G. Bildung von Cobyrinsäure mittels eines zellfreien Systems aus Clostridium tetanomorphum. Hoppe Seylers Z Physiol Chem. 1975 Sep;356(9):1353–1358. doi: 10.1515/bchm2.1975.356.2.1353. [DOI] [PubMed] [Google Scholar]
- Deeg R., Kriemler H. P., Bergmann K. H., Müller G. Zur Cobyrinsäure-Biosynthese. Neuartige, methylierte Hydroporphyrine und deren Bedeutung bei der Cobyrinsäure-Bildung. Hoppe Seylers Z Physiol Chem. 1977 Mar;358(3):339–352. [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Fenton W. A., Rosenberg L. E. Improved techniques for the extraction and chromatography of cobalamins. Anal Biochem. 1978 Oct 1;90(1):119–125. doi: 10.1016/0003-2697(78)90014-3. [DOI] [PubMed] [Google Scholar]
- Ferdinand W. The interpretation of non-hyperbolic rate curves for two-substrate enzymes. A possible mechanism for phosphofructokinase. Biochem J. 1966 Jan;98(1):278–283. doi: 10.1042/bj0980278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S. H. Amidation of, and (R)-1-amino-2-propanol attachment to, the corrin ring during vitamin B-12 biosynthesis by Clostridium tetanomorphum extracts. Biochim Biophys Acta. 1985 Sep 6;841(3):306–317. doi: 10.1016/0304-4165(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Friedmann H. C. Vitamin B 12 biosynthesis. Evidence for a new precursor vitamin B 12 5'-phosphate. J Biol Chem. 1968 Apr 25;243(8):2065–2075. [PubMed] [Google Scholar]
- Jeter R. M., Olivera B. M., Roth J. R. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984 Jul;159(1):206–213. doi: 10.1128/jb.159.1.206-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY E. P. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J Biol Chem. 1956 Sep;222(1):185–191. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Lim C. K., Rideout J. M., Wright D. J. High-performance liquid chromatography of naturally occurring 8-, 7-, 6-, 5- and 4-carboxylic porphyrin isomers. J Chromatogr. 1983 Dec 30;282:629–641. doi: 10.1016/s0021-9673(00)91640-6. [DOI] [PubMed] [Google Scholar]
- Rapp P. Amidierung von Corrinoidcarbonsäuren in Rohextrakten aus Propionibacterium shermanii. Hoppe Seylers Z Physiol Chem. 1973 Feb;354(2):136–140. [PubMed] [Google Scholar]
- Ronzio R. A., Barker H. A. Enzymic synthesis of guanosine diphosphate cobinamide by extracts of propionic acid bacteria. Biochemistry. 1967 Aug;6(8):2344–2354. doi: 10.1021/bi00860a009. [DOI] [PubMed] [Google Scholar]
- Rudolph F. B. Product inhibition and abortive complex formation. Methods Enzymol. 1979;63:411–436. doi: 10.1016/0076-6879(79)63018-5. [DOI] [PubMed] [Google Scholar]
- Scott A. I., Irwin A. J., Siegel L. M., Schoolery J. N. Sirohydrochlorin. Prosthetic group of a sulfite reductase enzyme and its role in the biosynthesis of vitamin B12. J Am Chem Soc. 1978 Jan 4;100(1):316–318. doi: 10.1021/ja00469a071. [DOI] [PubMed] [Google Scholar]
- Scott A. I., Kajiwara M., Santander P. J. Biosynthesis of vitamin B12: concerning the origin of the methine protons of the corrin nucleus. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6616–6618. doi: 10.1073/pnas.84.19.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. I., Townsend C. A., Okada K., Kajiwara M. Biosynthesis of corrins. I. Experiments with 14-C porphobilinogen and 14-C uroporphyrinogens. J Am Chem Soc. 1974 Dec 25;96(26):8054–8069. doi: 10.1021/ja00833a036. [DOI] [PubMed] [Google Scholar]
- Scott A. I., Townsend C. A., Okada K., Kajiwara M., Cushley R. J., Whitman P. J. Biosynthesis of corrins. II. Incorporation of 13C-labeled substrates in vitamins B12. J Am Chem Soc. 1974 Dec 25;96(26):8069–8080. doi: 10.1021/ja00833a037. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Francis J. E. Investigations of rat liver uroporphyrinogen decarboxylase. Comparisons of porphyrinogens I and III as substrates and the inhibition by porphyrins. Biochem J. 1981 Apr 1;195(1):241–250. doi: 10.1042/bj1950241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland P. M. Pharmacological and biochemical aspects of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase. Pharmacol Rev. 1982 Sep;34(3):223–253. [PubMed] [Google Scholar]