Abstract
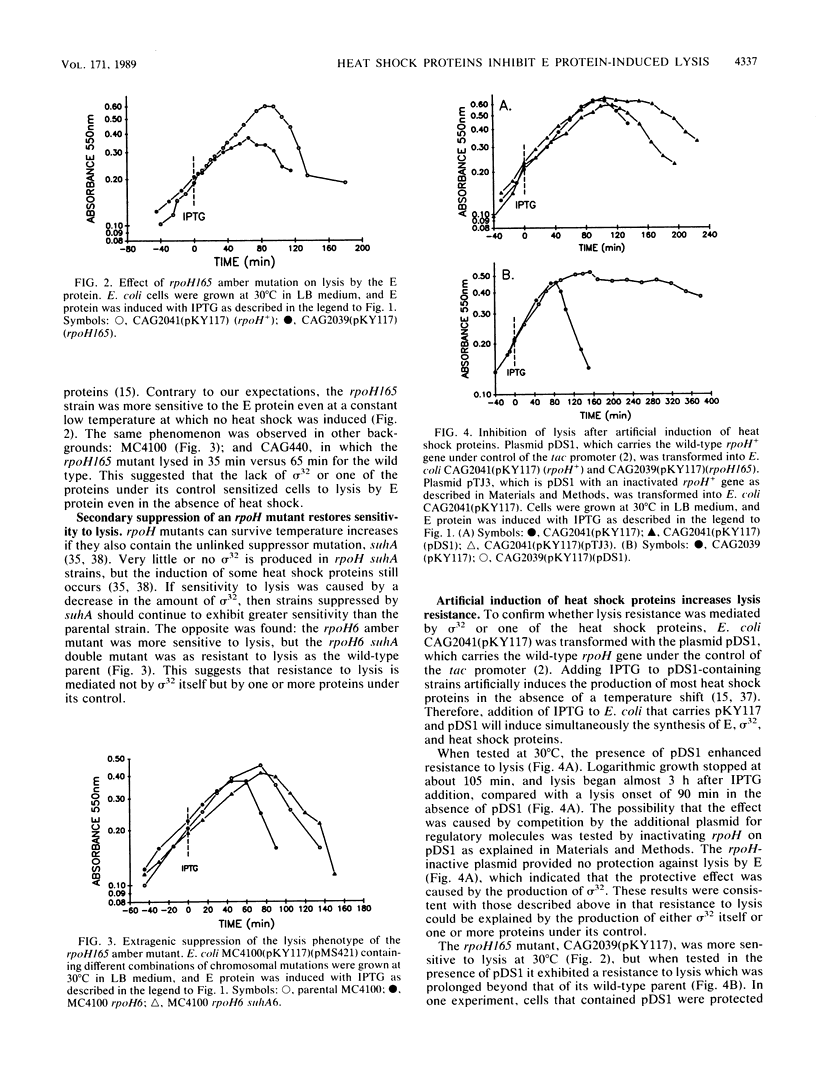
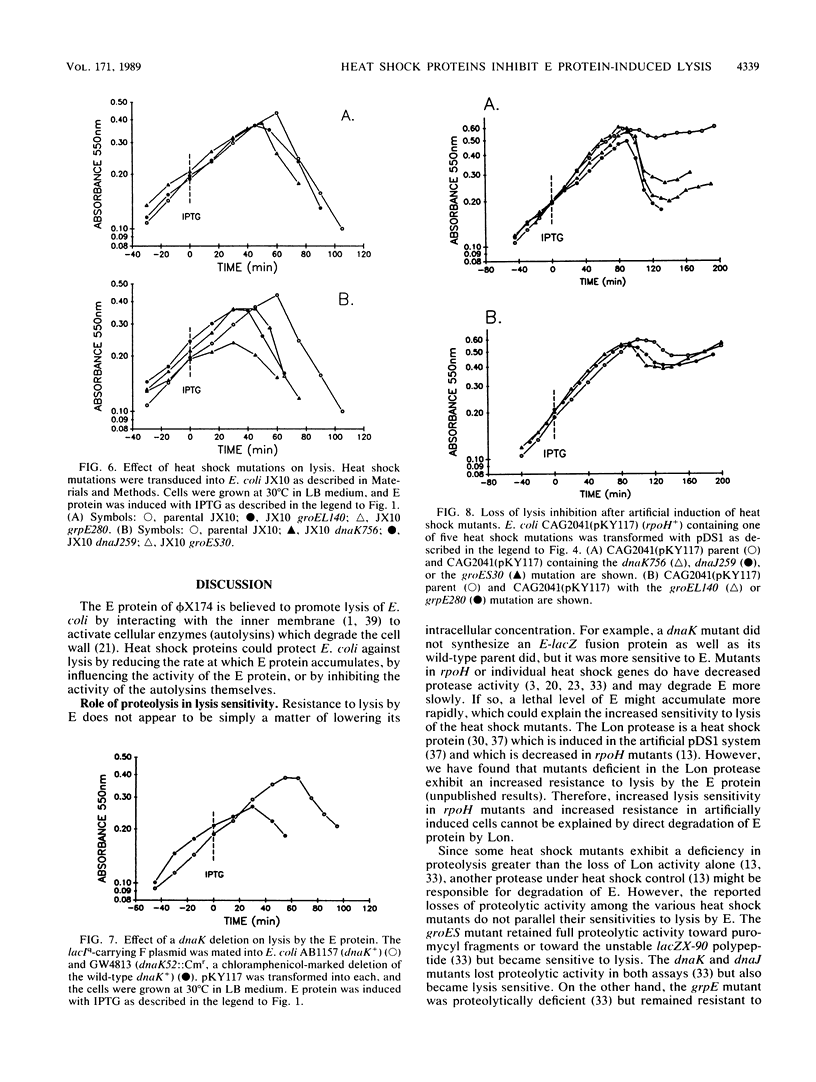
Lysis of Escherichia coli by the cloned E protein of bacteriophage phi X174 was more rapid than expected when bacteria were shifted from 30 to 42 degrees C at the time of E induction. Since such treatment also induces the heat shock response, we investigated the effect of heat shock proteins on lysis. An rpoH mutant was more sensitive to lysis by E, but a secondary suppressor mutation restored lysis resistance to parental levels, which suggests that the sigma 32 subunit itself did not directly increase lysis resistance. At 30 degrees C, mutants in five heat shock genes (dnaK, dnaJ, groEL, groES, and grpE) were more sensitive to lysis than were their wild-type parents. The magnitude of lysis sensitivity varied with mutation and strain background, with dnaK, dnaJ, and groES mutants consistently exhibiting the greatest sensitivities. Extended protection against lysis occurred when overproduction of heat shock proteins was induced artificially in cells that contained a plasmid with the rpoH+ gene under control of the tac promoter. This protective effect was completely abolished by mutations in dnaK, dnaJ, or groES but not by grpE or groEL mutations. Altered membrane behavior probably explains the contradiction whereby an actual temperature shift sensitized cells to lysis, but production of heat shock proteins exhibited protective effects. The results demonstrate that E-induced lysis can be divided into two distinct operations which may now be studied separately. They also emphasize a role for heat shock proteins under non-heat-shock conditions and suggest cautious interpretation of lysis phenomena in systems where E protein production is under control of a temperature-sensitive repressor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Young K., Garrett J., Altman R., Young R. Subcellular localization of lethal lysis proteins of bacteriophages lambda and phiX174. J Virol. 1985 Mar;53(3):1008–1011. doi: 10.1128/jvi.53.3.1008-1011.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl H., Echols H., Straus D. B., Court D., Crowl R., Georgopoulos C. P. Induction of the heat shock response of E. coli through stabilization of sigma 32 by the phage lambda cIII protein. Genes Dev. 1987 Mar;1(1):57–64. doi: 10.1101/gad.1.1.57. [DOI] [PubMed] [Google Scholar]
- Baker T. A., Grossman A. D., Gross C. A. A gene regulating the heat shock response in Escherichia coli also affects proteolysis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6779–6783. doi: 10.1073/pnas.81.21.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsi U., Henrich B., Lubitz W. Lysis of Escherichia coli by cloned phi X174 gene E depends on its expression. J Gen Microbiol. 1985 May;131(5):1107–1114. doi: 10.1099/00221287-131-5-1107. [DOI] [PubMed] [Google Scholar]
- Bochkareva E. S., Lissin N. M., Girshovich A. S. Transient association of newly synthesized unfolded proteins with the heat-shock GroEL protein. Nature. 1988 Nov 17;336(6196):254–257. doi: 10.1038/336254a0. [DOI] [PubMed] [Google Scholar]
- Buckley K. J., Hayashi M. Lytic activity localized to membrane-spanning region of phi X174 E protein. Mol Gen Genet. 1986 Jul;204(1):120–125. doi: 10.1007/BF00330198. [DOI] [PubMed] [Google Scholar]
- Crickmore N., Salmond G. P. The Escherichia coli heat shock regulatory gene is immediately downstream of a cell division operon: the fam mutation is allelic with rpoH. Mol Gen Genet. 1986 Dec;205(3):535–539. doi: 10.1007/BF00338094. [DOI] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N., Ishihama A. Heat-shock induction of RNA polymerase sigma-32 synthesis in Escherichia coli: transcriptional control and a multiple promoter system. Mol Gen Genet. 1987 Nov;210(1):10–15. doi: 10.1007/BF00337752. [DOI] [PubMed] [Google Scholar]
- Garrett J. M., Young R. Lethal action of bacteriophage lambda S gene. J Virol. 1982 Dec;44(3):886–892. doi: 10.1128/jvi.44.3.886-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J., Fusselman R., Hise J., Chiou L., Smith-Grillo D., Schulz J., Young R. Cell lysis by induction of cloned lambda lysis genes. Mol Gen Genet. 1981;182(2):326–331. doi: 10.1007/BF00269678. [DOI] [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Straus D. B., Walter W. A., Gross C. A. Sigma 32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987 Apr;1(2):179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Henrich B., Lubitz W., Plapp R. Lysis of Escherichia coli by induction of cloned phi X174 genes. Mol Gen Genet. 1982;185(3):493–497. doi: 10.1007/BF00334146. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Keller J. A., Simon L. D. Divergent effects of a dnaK mutation on abnormal protein degradation in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):31–41. doi: 10.1111/j.1365-2958.1988.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Lubitz W., Harkness R. E., Ishiguro E. E. Requirement for a functional host cell autolytic enzyme system for lysis of Escherichia coli by bacteriophage phi X174. J Bacteriol. 1984 Jul;159(1):385–387. doi: 10.1128/jb.159.1.385-387.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandecki W., Powell B. S., Mollison K. W., Carter G. W., Fox J. L. High-level expression of a gene encoding the human complement factor C5a in Escherichia coli. Gene. 1986;43(1-2):131–138. doi: 10.1016/0378-1119(86)90016-8. [DOI] [PubMed] [Google Scholar]
- Maratea D., Young K., Young R. Deletion and fusion analysis of the phage phi X174 lysis gene E. Gene. 1985;40(1):39–46. doi: 10.1016/0378-1119(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M. J., Gibbins J. R., Postgate J. R. A rapid and efficient method for plasmid transformation of Klebsiella pneumoniae and Escherichia coli. J Gen Microbiol. 1987 Aug;133(8):2053–2057. doi: 10.1099/00221287-133-8-2053. [DOI] [PubMed] [Google Scholar]
- Miki T., Orita T., Furuno M., Horiuchi T. Control of cell division by sex factor F in Escherichia coli. III. Participation of the groES (mopB) gene of the host bacteria. J Mol Biol. 1988 May 20;201(2):327–338. doi: 10.1016/0022-2836(88)90141-6. [DOI] [PubMed] [Google Scholar]
- Paek K. H., Walker G. C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987 Jan;169(1):283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: on the role of the S function in lysis. Virology. 1971 Mar;43(3):623–637. doi: 10.1016/0042-6822(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Walter W. A., Gross C. A. Escherichia coli heat shock gene mutants are defective in proteolysis. Genes Dev. 1988 Dec;2(12B):1851–1858. doi: 10.1101/gad.2.12b.1851. [DOI] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Tobe T., Kusukawa N., Yura T. Suppression of rpoH (htpR) mutations of Escherichia coli: heat shock response in suhA revertants. J Bacteriol. 1987 Sep;169(9):4128–4134. doi: 10.1128/jb.169.9.4128-4134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchido T., VanBogelen R. A., Neidhardt F. C. Heat shock response in Escherichia coli influences cell division. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6959–6963. doi: 10.1073/pnas.83.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Acton M. A., Neidhardt F. C. Induction of the heat shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev. 1987 Aug;1(6):525–531. doi: 10.1101/gad.1.6.525. [DOI] [PubMed] [Google Scholar]
- Wada C., Akiyama Y., Ito K., Yura T. Inhibition of F plasmid replication in htpR mutants of Escherichia coli deficient in sigma 32 protein. Mol Gen Genet. 1986 May;203(2):208–213. doi: 10.1007/BF00333956. [DOI] [PubMed] [Google Scholar]
- Young K. D., Young R. Lytic action of cloned phi X174 gene E. J Virol. 1982 Dec;44(3):993–1002. doi: 10.1128/jvi.44.3.993-1002.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


