Abstract
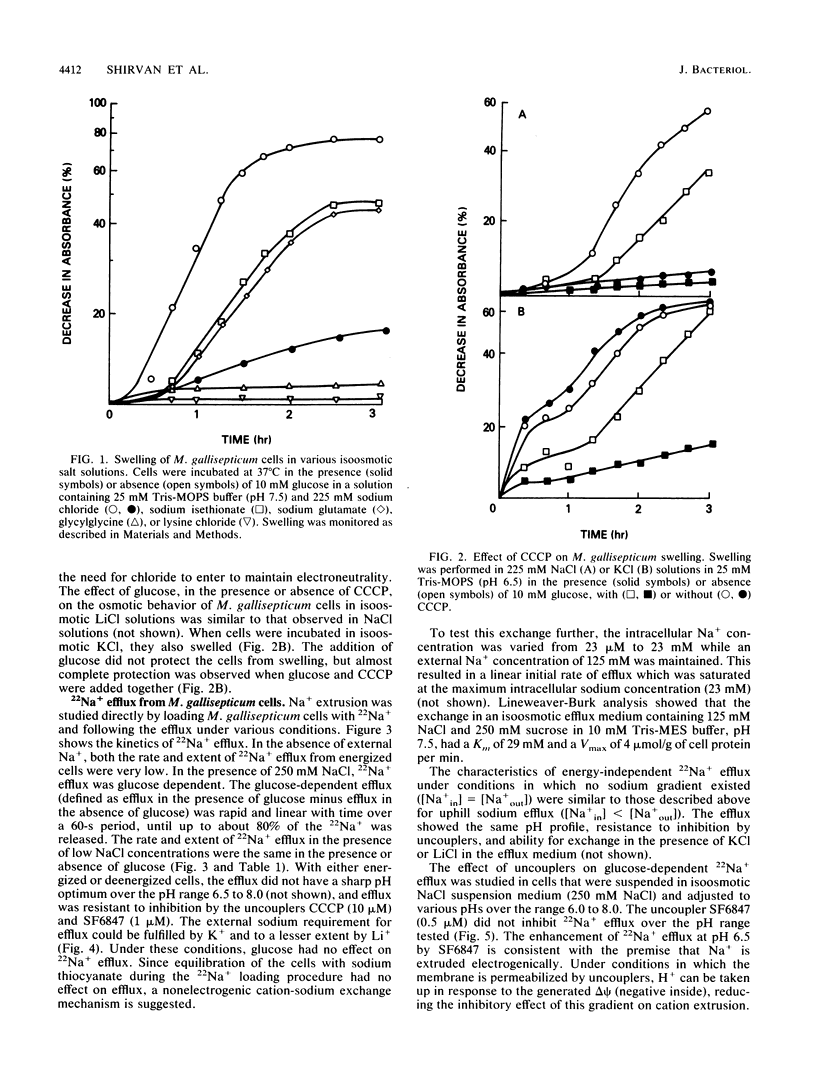
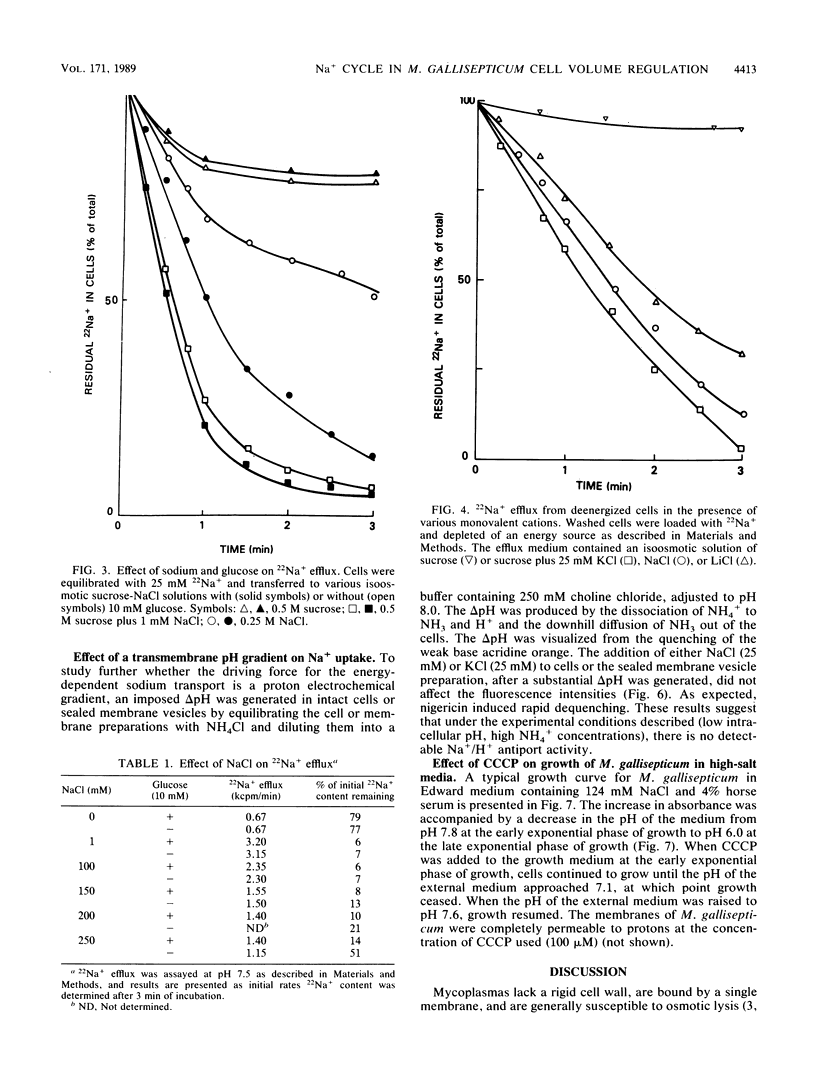
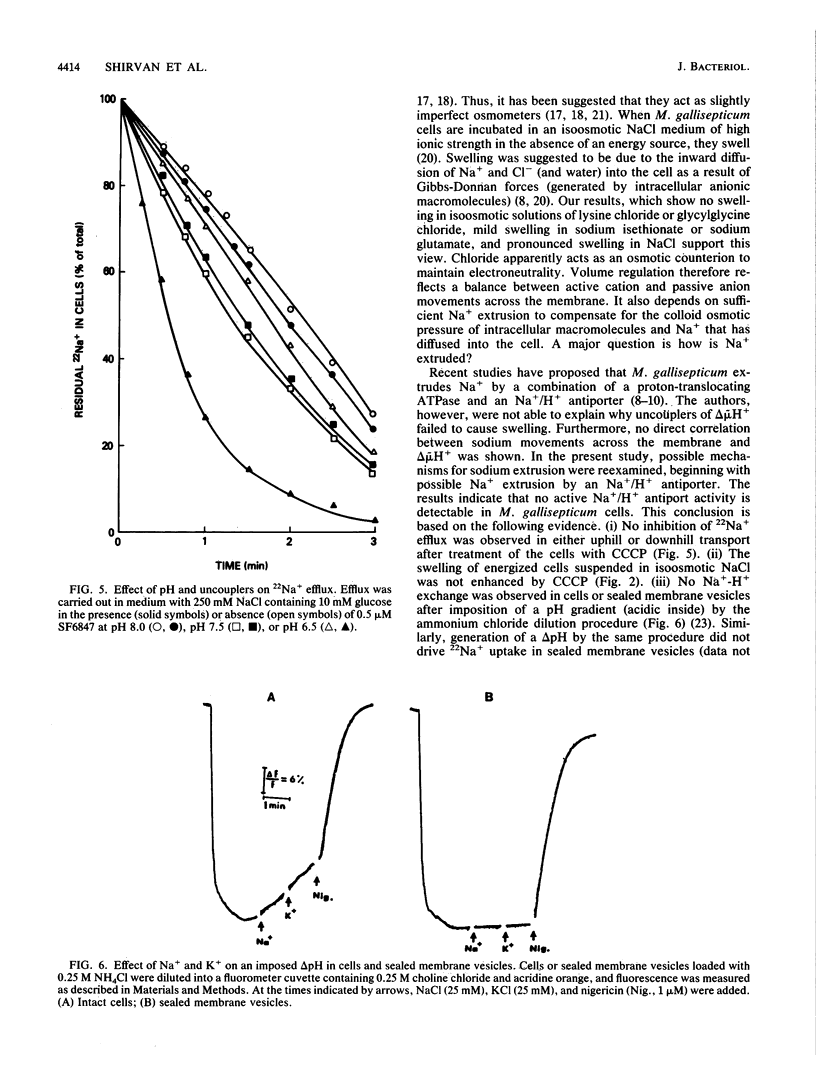
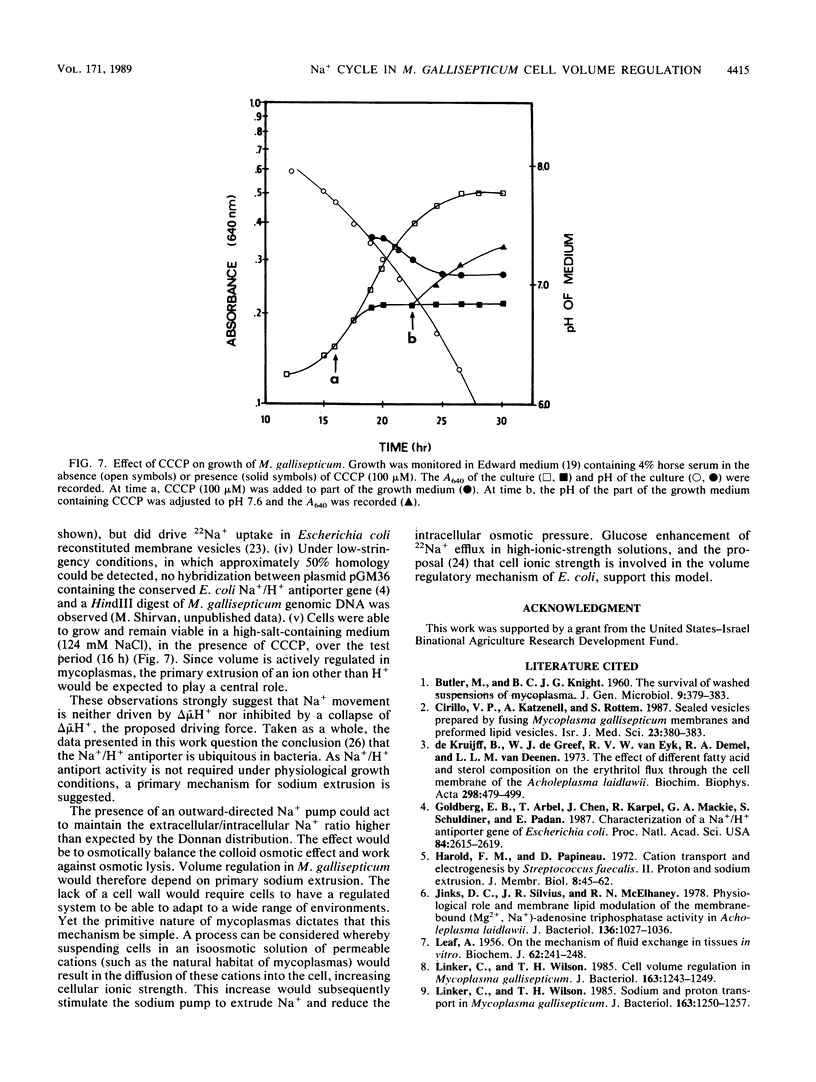
The mechanism for the extrusion of Na+ from Mycoplasma gallisepticum cells was examined. Na+ efflux from cells was studied by diluting 22Na+-loaded cells into an isoosmotic NaCl solution and measuring the residual 22Na+ in the cells. Uphill 22Na+ efflux was found to be glucose dependent and linear with time over a 60-s period and showed almost the same rate in the pH range of 6.5 to 8.0. 22Na+ efflux was markedly inhibited by dicyclohexylcarbodiimide (DCCD, 10 microM), but not by the proton-conducting ionophores SF6847 (0.5 microM) or carbonyl cyanide m-chlorophenylhydrazone (CCCP, 10 microM) over the entire pH range tested. An ammonium diffusion potential and a pH gradient were created by diluting intact cells or sealed membrane vesicles of M. gallisepticum loaded with NH4Cl into a choline chloride solution. The imposed H+ gradient (inside acid) was not affected by the addition of either NaCl or KCl to the medium. Dissipation of the proton motive force by CCCP had no effect on the growth of M. gallisepticum in the pH range of 7.2 to 7.8 in an Na+-rich medium. Additionally, energized M. gallisepticum cells were stable in an isoosmotic NaCl solution, even in the presence of proton conductors, whereas nonenergized cells tended to swell and lyse. These results show that in M. gallisepticum Na+ movement was neither driven nor inhibited by the collapse of the electrochemical gradient of H+, suggesting that in this organism Na+ is extruded by an electrogenic primary Na+ pump rather than by an Na+-H+ exchange system energized by the proton motive force.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cirillo V. P., Katzenell A., Rottem S. Sealed vesicles prepared by fusing Mycoplasma gallisepticum membranes and preformed lipid vesicles. Isr J Med Sci. 1987 May;23(5):380–383. [PubMed] [Google Scholar]
- Goldberg E. B., Arbel T., Chen J., Karpel R., Mackie G. A., Schuldiner S., Padan E. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(9):2615–2619. doi: 10.1073/pnas.84.9.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol. 1972;8(1):45–62. doi: 10.1007/BF01868094. [DOI] [PubMed] [Google Scholar]
- Jinks D. C., Silvius J. R., McElhaney R. N. Physiological role and membrane lipid modulation of the membrane-bound (Mg2+, na+)-adenosine triphosphatase activity in Acholeplasma laidlawii. J Bacteriol. 1978 Dec;136(3):1027–1036. doi: 10.1128/jb.136.3.1027-1036.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAF A. On the mechanism of fluid exchange of tissues in vitro. Biochem J. 1956 Feb;62(2):241–248. doi: 10.1042/bj0620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linker C., Wilson T. H. Cell volume regulation in Mycoplasma gallisepticum. J Bacteriol. 1985 Sep;163(3):1243–1249. doi: 10.1128/jb.163.3.1243-1249.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C., Wilson T. H. Characterization and solubilization of the membrane-bound ATPase of Mycoplasma gallisepticum. J Bacteriol. 1985 Sep;163(3):1258–1262. doi: 10.1128/jb.163.3.1258-1262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C., Wilson T. H. Sodium and proton transport in Mycoplasma gallisepticum. J Bacteriol. 1985 Sep;163(3):1250–1257. doi: 10.1128/jb.163.3.1250-1257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Hsu C., Rosen B. P. Cation/proton antiport systems in Escherichia coli. Solubilization and reconstitution of delta pH-driven sodium/proton and calcium/proton antiporters. J Biol Chem. 1986 Jan 15;261(2):678–683. [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Linker C., Wilson T. H. Proton motive force across the membrane of Mycoplasma gallisepticum and its possible role in cell volume regulation. J Bacteriol. 1981 Mar;145(3):1299–1304. doi: 10.1128/jb.145.3.1299-1304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Verkleij A. J. Possible association of segregated lipid domains of Mycoplasma gallisepticum membranes with cell resistance to osmotic lysis. J Bacteriol. 1982 Jan;149(1):338–345. doi: 10.1128/jb.149.1.338-345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Fishkes H. Sodium-proton antiport in isolated membrane vesicles of Escherichia coli. Biochemistry. 1978 Feb 21;17(4):706–711. doi: 10.1021/bi00597a023. [DOI] [PubMed] [Google Scholar]
- Shirvan M. H., Schuldiner S., Rottem S. Control of sodium fluxes in Mycoplasma gallisepticum. Isr J Med Sci. 1987 May;23(5):384–388. [PubMed] [Google Scholar]
- WILSON T. H. Ionic permeability and osmotic swelling of cells. Science. 1954 Jul 16;120(3107):104–105. doi: 10.1126/science.120.3107.104. [DOI] [PubMed] [Google Scholar]
- Wilson T. H., Lin E. C. Evolution of membrane bioenergetics. J Supramol Struct. 1980;13(4):421–446. doi: 10.1002/jss.400130403. [DOI] [PubMed] [Google Scholar]
- de Kruyff B., de Greef W. J., van Eyk R. V., Demel R. A., van Deenen L. L. The effect of different fatty acid and sterol composition on the erythritol flux through the cell membrane of Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Mar 16;298(2):479–499. doi: 10.1016/0005-2736(73)90375-1. [DOI] [PubMed] [Google Scholar]


