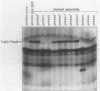
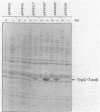
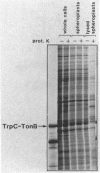
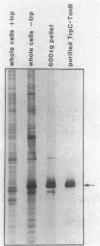
Abstract
We have developed a selection for mutations in a trpC-tonB gene fusion that takes advantage of the properties of the plasmid-encoded TrpC-TonB hybrid protein. The TrpC-TonB hybrid protein consists of amino acids 1 through 25 of the normally cytoplasmic protein, TrpC, fused to amino acids 12 through 239 of TonB. It is expressed from the trp promoter and is regulated by the trpR gene and the presence or absence of tryptophan. Under repressing conditions in the presence of tryptophan, the trpC-tonB gene can restore phi 80 sensitivity to a tonB deletion mutant, which indicates that TrpC-TonB can be exported and is functional. High-level expression of TrpC-TonB protein in the absence of tryptophan results in virtually immediate cessation of growth for strains carrying the trpC-tonB plasmid. By selecting for survivors of the induced growth inhibition (overproduction lethality), we have isolated a variety of mutations. Many of the mutations decrease expression of the TrpC-TonB protein, as expected. In addition, three independently isolated mutants expressing normal levels of TrpC-TonB protein result in a Gly----Asp substitution within the hydrophobic amino terminus of TonB. The mutant proteins are designated TrpC-TonBG26D. The mutations are suppressed by prlA alleles, known to suppress export (signal sequence) mutations. TrpC-TonB proteins carrying the Gly----Asp substitution accumulate in the cytoplasm. We conclude that the Gly----Asp substitution is an export mutation. TrpC-TonBG26D protein has been purified and used to raise polyclonal antibodies that specifically recognize both TrpC-TonB protein and wild-type TonB protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankaitis V. A., Bassford P. J., Jr The synthesis of export-defective proteins can interfere with normal protein export in Escherichia coli. J Biol Chem. 1984 Oct 10;259(19):12193–12200. [PubMed] [Google Scholar]
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Silhavy T. J. Mutations affecting localization of an Escherichia coli outer membrane protein, the bacteriophage lambda receptor. J Mol Biol. 1980 Jul 25;141(1):63–90. doi: 10.1016/s0022-2836(80)80029-5. [DOI] [PubMed] [Google Scholar]
- Horabin J. I., Webster R. E. An amino acid sequence which directs membrane insertion causes loss of membrane potential. J Biol Chem. 1988 Aug 15;263(23):11575–11583. [PubMed] [Google Scholar]
- Inouye S., Vlasuk G. P., Hsiung H., Inouye M. Effects of mutations at glycine residues in the hydrophobic region of the Escherichia coli prolipoprotein signal peptide on the secretion across the membrane. J Biol Chem. 1984 Mar 25;259(6):3729–3733. [PubMed] [Google Scholar]
- Isackson P. J., Bertrand K. P. Dominant negative mutations in the Tn10 tet repressor: evidence for use of the conserved helix-turn-helix motif in DNA binding. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6226–6230. doi: 10.1073/pnas.82.18.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., McElhaney G. Outer membrane-dependent transport systems in Escherichia coli: turnover of TonB function. J Bacteriol. 1978 Jun;134(3):1020–1029. doi: 10.1128/jb.134.3.1020-1029.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pollitt N. S., Inouye M. Synthesis of an Escherichia coli protein carrying a signal peptide mutation causes depolarization of the cytoplasmic membrane potential. J Bacteriol. 1988 May;170(5):2051–2055. doi: 10.1128/jb.170.5.2051-2055.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Good R. F. A bidirectional rho-independent transcription terminator between the E. coli tonB gene and an opposing gene. Cell. 1985 Jun;41(2):577–585. doi: 10.1016/s0092-8674(85)80030-1. [DOI] [PubMed] [Google Scholar]
- Postle K., Good R. F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Skare J. T. Escherichia coli TonB protein is exported from the cytoplasm without proteolytic cleavage of its amino terminus. J Biol Chem. 1988 Aug 5;263(22):11000–11007. [PubMed] [Google Scholar]
- SHEDLOVSKY A., BRENNER S. A CHEMICAL BASIS FOR THE HOST-INDUCED MODIFICATION OF T-EVEN BACTERIOPHAGES. Proc Natl Acad Sci U S A. 1963 Aug;50:300–305. doi: 10.1073/pnas.50.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stader J., Benson S. A., Silhavy T. J. Kinetic analysis of lamB mutants suggests the signal sequence plays multiple roles in protein export. J Biol Chem. 1986 Nov 15;261(32):15075–15080. [PubMed] [Google Scholar]
- Wu A. M., Christie G. E., Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]