Abstract
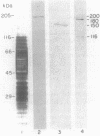
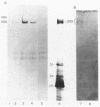
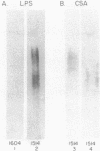
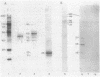
A cell surface antigen complex from Zwittergent-solubilized Myxococcus xanthus has been purified by immunoaffinity chromatography with monoclonal antibody (MAb) 1604 and by subsequent gel filtration. We propose that the cell surface antigen (CSA) 1604 complex participates in intercellular interactions. The apparent total molecular mass of the CSA 1604 complex is 200 kilodaltons (kDa), as determined by gel filtration and by electrophoresis and Western immunoblot probing with MAb 1604. The antigen epitope recognized by MAb 1604 is on a 51-kDa polypeptide. The CSA complex also contains 14% neutral carbohydrate and a 23-kDa polypeptide that lacks the 1604 epitope. The carbohydrate is most likely part of a lipopolysaccharide (LPS) associated with the CSA, because an MAb recognizing an O antigen epitope from the LPS of M. xanthus also reacted with CSA 1604 on Western immunoblots. Thus, the 200-kDa CSA complex consists of 97 +/- 6 kDa of protein and many associated LPS molecules. The LPS evidently produces the multiplicity of bands observed on Western immunoblots between 100 and 200 kDa. The association with LPS may contribute to the negative charge of the CSA 1604 complex, which has a pI of 4.3. The CSA was clustered on the surface of intact M. xanthus cells after labeling with MAb 1604 and immunogold. Furthermore, fractionation studies indicated that cells grown on a plastic surface had 50% of their total CSA 1604 in the cytosol, 39% in the membrane fraction, and 8% in the periplasm. Saturable binding studies with 125I-MAb 1604 indicated that there were 2,400 CSA 1604 sites per cell. The Kd for MAb 1604 binding to the cell was 9 nM.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson S. A., Bremer E., Silhavy T. J. Intragenic regions required for LamB export. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3830–3834. doi: 10.1073/pnas.81.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley A. T., Klebba P. E. Effect of lipopolysaccharide structure on reactivity of antiporin monoclonal antibodies with the bacterial cell surface. J Bacteriol. 1988 Mar;170(3):1063–1068. doi: 10.1128/jb.170.3.1063-1068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P. Studies on gliding motility in Myxococcus xanthus. Arch Microbiol. 1974;99(3):271–280. doi: 10.1007/BF00696242. [DOI] [PubMed] [Google Scholar]
- Cumsky M. G., Zusman D. R. Binding properties of myxobacterial hemagglutinin. J Biol Chem. 1981 Dec 10;256(23):12596–12599. [PubMed] [Google Scholar]
- Cumsky M., Zusman D. R. Myxobacterial hemagglutinin: a development-specific lectin of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5505–5509. doi: 10.1073/pnas.76.11.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C., Greggs R., Duggan K., Stubbs J., Horwitz A. Adhesive multiplicity in the interaction of embryonic fibroblasts and myoblasts with extracellular matrices. J Cell Biol. 1984 Oct;99(4 Pt 1):1398–1404. doi: 10.1083/jcb.99.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M., Kaiser D. Cell interactions in myxobacterial growth and development. Science. 1985 Oct 4;230(4721):18–24. doi: 10.1126/science.3929384. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fink J. M., Kalos M., Zissler J. F. Isolation of cell surface antigen mutants of Myxococcus xanthus by use of monoclonal antibodies. J Bacteriol. 1989 Apr;171(4):2033–2041. doi: 10.1128/jb.171.4.2033-2041.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. M., Zissler J. F. Characterization of lipopolysaccharide from Myxococcus xanthus by use of monoclonal antibodies. J Bacteriol. 1989 Apr;171(4):2028–2032. doi: 10.1128/jb.171.4.2028-2032.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. S., Dworkin M. Cell surface antigens during submerged development of Myxococcus xanthus examined with monoclonal antibodies. J Bacteriol. 1986 Nov;168(2):505–511. doi: 10.1128/jb.168.2.505-511.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J. S., Jarvis B. W., Dworkin M. Inhibition of development in Myxococcus xanthus by monoclonal antibody 1604. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4505–4508. doi: 10.1073/pnas.84.13.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J., Stellwag E., Dworkin M. Monoclonal antibodies against cell-surface antigens of developing cells of Myxococcus xanthus. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):11–18. doi: 10.1016/s0769-2609(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Helenius A., McCaslin D. R., Fries E., Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- Hjelmeland L. M., Chrambach A. Solubilization of functional membrane proteins. Methods Enzymol. 1984;104:305–318. doi: 10.1016/s0076-6879(84)04097-0. [DOI] [PubMed] [Google Scholar]
- Hjelmeland L. M., Klee W. A., Osborne J. C., Jr A new class of nonionic detergents with a gluconamide polar group. Anal Biochem. 1983 Apr 15;130(2):485–490. doi: 10.1016/0003-2697(83)90621-8. [DOI] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Biosynthesis and self-assembly of protein S, a development-specific protein of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):209–213. doi: 10.1073/pnas.76.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen G. R., Dworkin M. Cell-cell interactions in developmental lysis of Myxococcus xanthus. Dev Biol. 1985 Nov;112(1):194–202. doi: 10.1016/0012-1606(85)90133-2. [DOI] [PubMed] [Google Scholar]
- Jarvis B. W., Dworkin M. Role of Myxococcus xanthus cell surface antigen 1604 in development. J Bacteriol. 1989 Sep;171(9):4667–4673. doi: 10.1128/jb.171.9.4667-4673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982 Jul;151(1):458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Storm D. R. Detection of calcium-dependent regulatory protein binding components using 125I-labeled calcium-dependent regulatory protein. J Biol Chem. 1978 May 25;253(10):3374–3377. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Louis C. F., Maffitt M., Jarvis B. Factors that modify the molecular size of phospholamban, the 23,000-dalton cardiac sarcoplasmic reticulum phosphoprotein. J Biol Chem. 1982 Dec 25;257(24):15182–15186. [PubMed] [Google Scholar]
- Maeba P. Y. Isolation of a surface glycoprotein from Myxococcus xanthus. J Bacteriol. 1986 May;166(2):644–650. doi: 10.1128/jb.166.2.644-650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Millán J. L., Stigbrand T. Antigenic determinants of human placental and testicular placental-like alkaline phosphatases as mapped by monoclonal antibodies. Eur J Biochem. 1983 Oct 17;136(1):1–7. doi: 10.1111/j.1432-1033.1983.tb07697.x. [DOI] [PubMed] [Google Scholar]
- Müller K. H., Trust T. J., Kay W. W. Unmasking of bacteriophage Mu lipopolysaccharide receptors in Salmonella enteritidis confers sensitivity to Mu and permits Mu mutagenesis. J Bacteriol. 1988 Mar;170(3):1076–1081. doi: 10.1128/jb.170.3.1076-1081.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. T., Lowrey C., Decker C., Tovar A., Damsky C., Buck C., Horwitz A. F. A monoclonal antibody detaches embryonic skeletal muscle from extracellular matrices. J Cell Biol. 1982 Nov;95(2 Pt 1):654–666. doi: 10.1083/jcb.95.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. R., Cumsky M. G., Zusman D. R. Localization of myxobacterial hemagglutinin in the periplasmic space and on the cell surface of Myxococcus xanthus during developmental aggregation. J Biol Chem. 1981 Dec 10;256(23):12589–12595. [PubMed] [Google Scholar]
- Nelson D. R., Zusman D. R. Transport and localization of protein S, a spore coat protein, during fruiting body formation by Myxococcus xanthus. J Bacteriol. 1983 May;154(2):547–553. doi: 10.1128/jb.154.2.547-553.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Dworkin M. Separation and properties of the cytoplasmic and outer membranes of vegetative cells of Myxococcus xanthus. J Bacteriol. 1980 Feb;141(2):914–927. doi: 10.1128/jb.141.2.914-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko S. M. Methylation of macromolecules during development in Myxococcus xanthus. J Bacteriol. 1985 Nov;164(2):495–500. doi: 10.1128/jb.164.2.495-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocque W. J., Coughlin R. T., McGroarty E. J. Lipopolysaccharide tightly bound to porin monomers and trimers from Escherichia coli K-12. J Bacteriol. 1987 Sep;169(9):4003–4010. doi: 10.1128/jb.169.9.4003-4010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe R., Robins R. A., Laxton R. R., Baldwin R. W. Kinetics of divalent monoclonal antibody binding to tumour cell surface antigens using flow cytometry: standardization and mathematical analysis. Mol Immunol. 1985 Jan;22(1):11–21. doi: 10.1016/0161-5890(85)90029-x. [DOI] [PubMed] [Google Scholar]
- Schenkein I., Levy M., Uhr J. W. The use of glucose oxidase as a generator of H 2 O 2 in the enzymatic radioiodination of components of cell surfaces. Cell Immunol. 1972 Nov;5(3):490–493. doi: 10.1016/0008-8749(72)90076-7. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W., Thomson S. Comparison of polysaccharides produced by Myxococcus strains. J Gen Microbiol. 1975 Jul;89(1):124–132. doi: 10.1099/00221287-89-1-124. [DOI] [PubMed] [Google Scholar]
- Tortorello M. L., Dunny G. M. Identification of multiple cell surface antigens associated with the sex pheromone response of Streptococcus faecalis. J Bacteriol. 1985 Apr;162(1):131–137. doi: 10.1128/jb.162.1.131-137.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P., Letoffe S., Schwartz M. Characterization of Erwinia chrysanthemi extracellular proteases: cloning and expression of the protease genes in Escherichia coli. J Bacteriol. 1987 Nov;169(11):5046–5053. doi: 10.1128/jb.169.11.5046-5053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B. F., Dworkin M. Comparative intermediary metabolism of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1968 Nov;96(5):1465–1473. doi: 10.1128/jb.96.5.1465-1473.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. M., Siu C. H. Cloning of cDNA for the contact site A glycoprotein of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4248–4252. doi: 10.1073/pnas.83.12.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]