Abstract
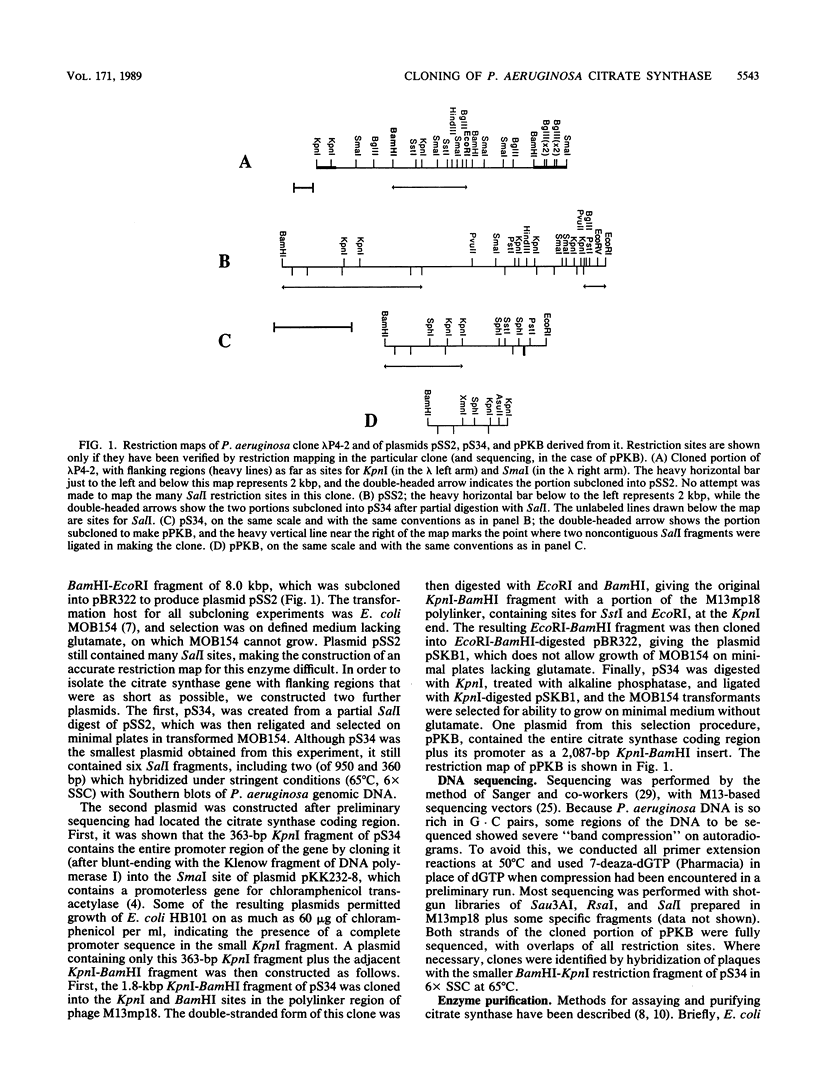
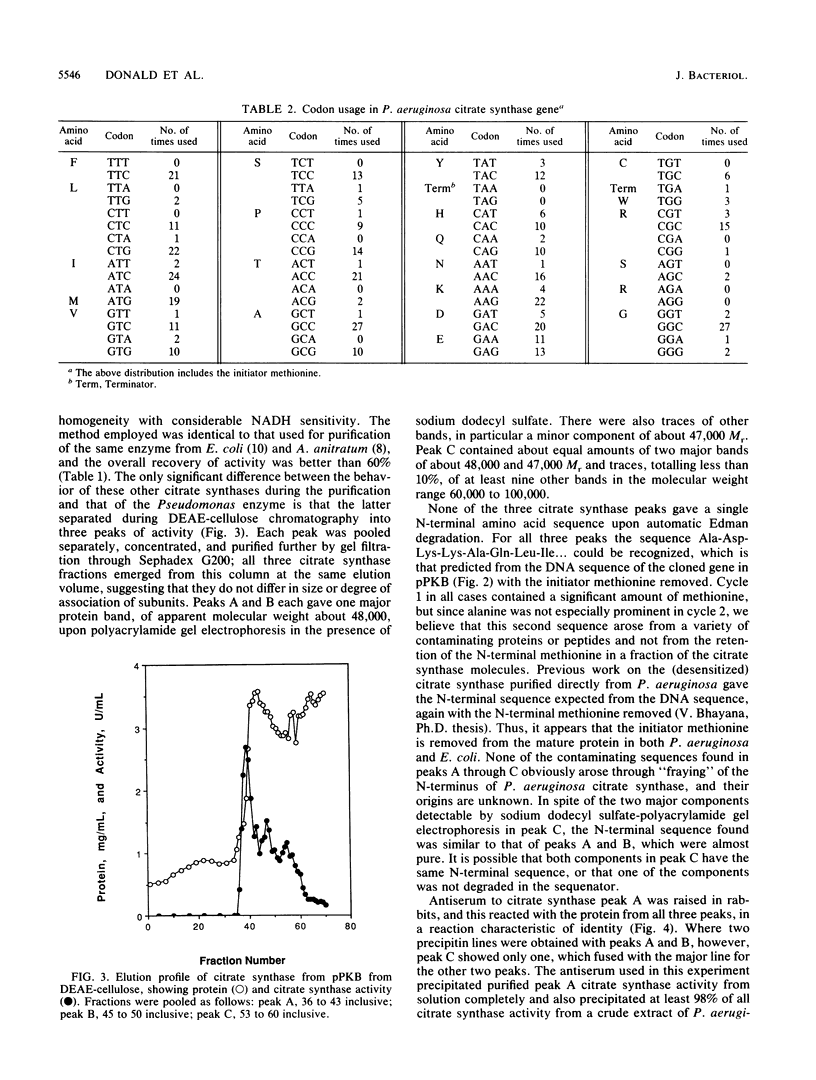
The structural gene for the allosteric citrate synthase of Pseudomonas aeruginosa has been cloned from a genomic library by using the Escherichia coli citrate synthase gene as a hybridization probe under conditions of reduced stringency. Subcloning of portions of the original 10-kilobase-pair (kbp) clone led to isolation of the structural gene, with its promoter, within a 2,083-bp length of DNA flanked by sites for KpnI and BamHI. The nucleotide sequence of this fragment is presented; the inferred amino acid sequence was 70 and 76% identical, respectively, with the citrate synthase sequences from E. coli and Acinetobacter anitratum, two other gram-negative bacteria. DEAE-cellulose chromatography of P. aeruginosa citrate synthase from an E. coli host harboring the cloned P. aeruginosa gene gave three peaks of activity. All three enzyme peaks had subunit molecular weights of 48,000; the proteins were identical by immunological criteria and very similar in kinetics of substrate saturation and NADH inhibition. Because the cloned gene contained only one open reading frame large enough to encode a polypeptide of such a size, the three peaks must represent different forms of the same protein. A portion of the cloned P. aeruginosa gene was used as a hybridization probe under stringent conditions to identify highly homologous sequences in genomic DNA of a second strain classified as P. aeruginosa and isolates of P. putida, P. stutzeri, and P. alcaligenes. When crude extracts of each of these four isolates were mixed with antiserum raised against purified P. aeruginosa citrate synthase, however, only the P. alcaligenes extract cross-reacted.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. H., Duckworth H. W. In vitro mutagenesis of Escherichia coli citrate synthase to clarify the locations of ligand binding sites. J Biol Chem. 1988 Feb 15;263(5):2163–2169. [PubMed] [Google Scholar]
- Bhayana V., Duckworth H. W. Amino acid sequence of Escherichia coli citrate synthase. Biochemistry. 1984 Jun 19;23(13):2900–2905. doi: 10.1021/bi00308a008. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Parmelee D. C., Kumar S., Walsh K. A., Titani K. Complete amino acid sequence of porcine heart citrate synthase. Biochemistry. 1982 Apr 27;21(9):2028–2036. doi: 10.1021/bi00538a009. [DOI] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Danson M. J., Weitzman P. D. Functional groups in the activity and regulation of Escherichia coli citrate synthase. Biochem J. 1973 Nov;135(3):513–524. doi: 10.1042/bj1350513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald L. J., Duckworth H. W. Expression and base sequence of the citrate synthase gene of Acinetobacter anitratum. Biochem Cell Biol. 1987 Nov;65(11):930–938. doi: 10.1139/o87-121. [DOI] [PubMed] [Google Scholar]
- Donald L. J., Duckworth H. W. Molecular cloning of the structural gene for Acinetobacter citrate synthase. Biochem Biophys Res Commun. 1986 Dec 15;141(2):797–803. doi: 10.1016/s0006-291x(86)80243-1. [DOI] [PubMed] [Google Scholar]
- Duckworth H. W., Anderson D. H., Bell A. W., Donald L. J., Chu A. L., Brayer G. D. Structural basis for regulation in gram-negative bacterial citrate synthases. Biochem Soc Symp. 1987;54:83–92. [PubMed] [Google Scholar]
- Duckworth H. W., Bell A. W. Large-scale production of citrate synthase from a cloned gene. Can J Biochem. 1982 Dec;60(12):1143–1147. doi: 10.1139/o82-146. [DOI] [PubMed] [Google Scholar]
- Flechtner V. R., Hanson R. S. Regulation of the tricarboxylic acid cycle in bacteria. A comparison of citrate synthases from different bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):253–264. doi: 10.1016/0304-4165(70)90114-5. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Harford S., Weitzman P. D. Evidence of isosteric and allosteric nucleotide inhibition of citrate synthease from multiple-inhibition studies. Biochem J. 1975 Nov;151(2):455–458. doi: 10.1042/bj1510455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa A. I., Massarini E., Cazzulo J. J. Purification and some properties of the citrate synthase from a marine Pseudomonas. Can J Microbiol. 1978 Mar;24(3):215–221. doi: 10.1139/m78-039. [DOI] [PubMed] [Google Scholar]
- Inouye S., Asai Y., Nakazawa A., Nakazawa T. Nucleotide sequence of a DNA segment promoting transcription in Pseudomonas putida. J Bacteriol. 1986 Jun;166(3):739–745. doi: 10.1128/jb.166.3.739-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarini E., Cazzulo J. J. Two forms of citrate synthase in a marine Pseudomonad. FEBS Lett. 1975 Sep 15;57(2):134–138. doi: 10.1016/0014-5793(75)80701-0. [DOI] [PubMed] [Google Scholar]
- Mermod N., Lehrbach P. R., Reineke W., Timmis K. N. Transcription of the TOL plasmid toluate catabolic pathway operon of Pseudomonas putida is determined by a pair of co-ordinately and positively regulated overlapping promoters. EMBO J. 1984 Nov;3(11):2461–2466. doi: 10.1002/j.1460-2075.1984.tb02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. G., Weitzman P. D. Molecular size diversity of citrate synthases from Pseudomonas species. J Gen Microbiol. 1986 Mar;132(3):737–742. doi: 10.1099/00221287-132-3-737. [DOI] [PubMed] [Google Scholar]
- Morse D., Duckworth H. W. A comparison of the citrate synthases of Escherichia coli and Acinetobacter anitratum. Can J Biochem. 1980 Sep;58(9):696–706. doi: 10.1139/o80-098. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Remington S., Wiegand G., Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111–152. doi: 10.1016/0022-2836(82)90452-1. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin J. L., Skinner E. R., Seshadri H. S. Studies on an acidic glycoprotein-containing fraction isolated from guinea-pig serum. Biochem J. 1964 Feb;90(2):316–330. doi: 10.1042/bj0900316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talgoy M. M., Bell A. W., Duckworth H. W. The reactions of Escherichia coli citrate synthase with the sulfhydryl reagents 5,5'-dithiobis-(2-nitrobenzoic acid) and 4,4'-dithiodipyridine. Can J Biochem. 1979 Jun;57(6):822–833. doi: 10.1139/o79-102. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Viebrock A., Zumft W. G. Molecular cloning, heterologous expression, and primary structure of the structural gene for the copper enzyme nitrous oxide reductase from denitrifying Pseudomonas stutzeri. J Bacteriol. 1988 Oct;170(10):4658–4668. doi: 10.1128/jb.170.10.4658-4668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman P. D., Danson M. J. Citrate synthase. Curr Top Cell Regul. 1976;10:161–204. doi: 10.1016/b978-0-12-152810-2.50011-5. [DOI] [PubMed] [Google Scholar]
- Wiegand G., Remington S. J. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15:97–117. doi: 10.1146/annurev.bb.15.060186.000525. [DOI] [PubMed] [Google Scholar]
- Wood D. O., Williamson L. R., Winkler H. H., Krause D. C. Nucleotide sequence of the Rickettsia prowazekii citrate synthase gene. J Bacteriol. 1987 Aug;169(8):3564–3572. doi: 10.1128/jb.169.8.3564-3572.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak D. J., Cram D. C., Daniels C. J., Galloway D. R. Nucleotide sequence and characterization of toxR: a gene involved in exotoxin A regulation in Pseudomonas aeruginosa. Nucleic Acids Res. 1987 Mar 11;15(5):2123–2135. doi: 10.1093/nar/15.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]