Abstract
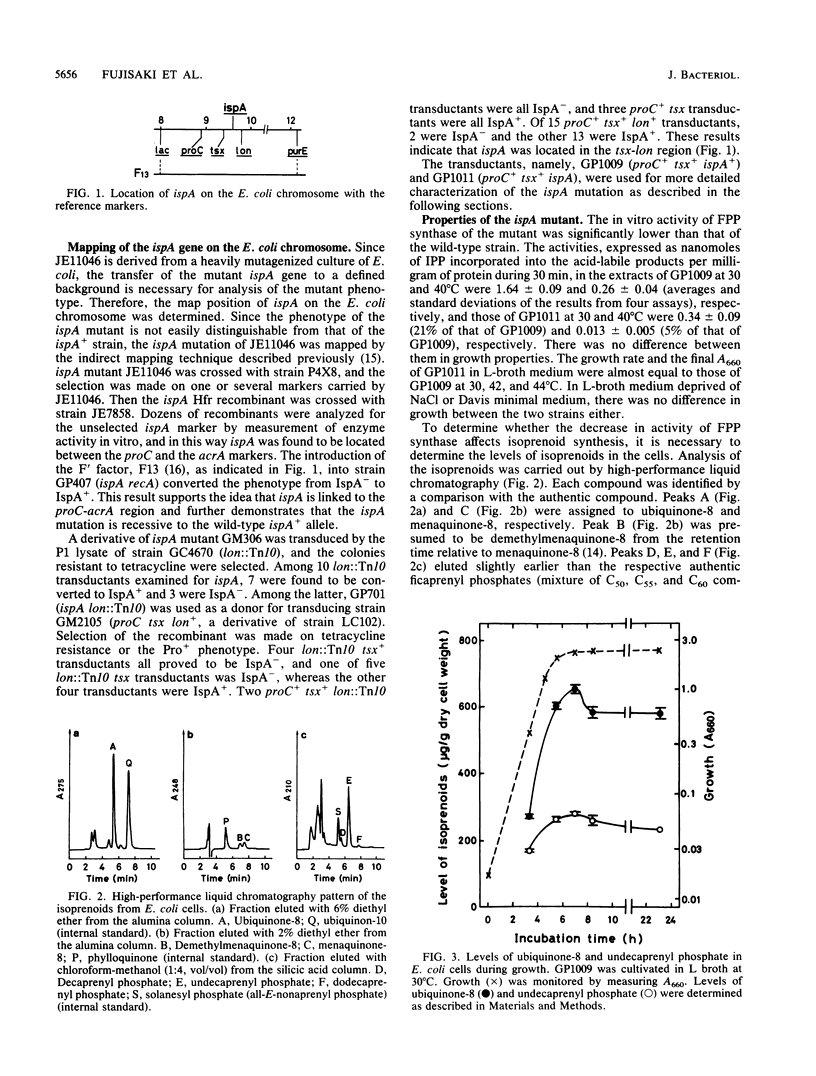
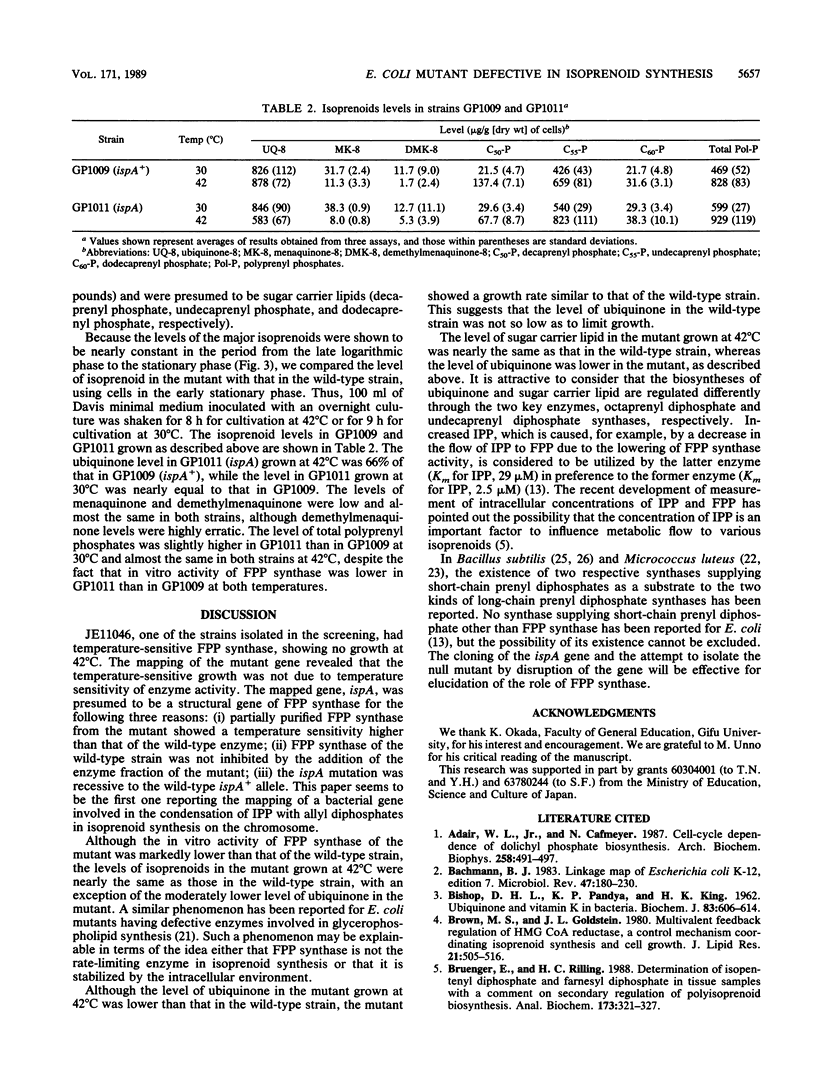
The screening of a collection of highly mutagenized strains of Escherichia coli for defects in isoprenoid synthesis led to the isolation of a mutant that had temperature-sensitive farnesyl diphosphate synthase. The defective gene, named ispA, was mapped at about min 10 on the E. coli chromosome, and the gene order was shown to be tsx-ispA-lon. The mutant ispA gene was transferred to the E. coli strain with a defined genetic background by P1 transduction for investigation of its function. The in vitro activity of farnesyl diphosphate synthase of the mutant was 21% of that of the wild-type strain at 30 degrees C and 5% of that at 40 degrees C. At 42 degrees C the ubiquinone level was lower (66% of normal) in the mutant than in the wild-type strain, whereas at 30 degrees C the level in the mutant was almost equal to that in the wild-type strain. The polyprenyl phosphate level was slightly higher in the mutant than in the wild-type strain at 30 degrees C and almost the same in both strains at 42 degrees C. The mutant had no obvious phenotype regarding its growth properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair W. L., Jr, Cafmeyer N. Cell-cycle dependence of dolichyl phosphate biosynthesis. Arch Biochem Biophys. 1987 Nov 1;258(2):491–497. doi: 10.1016/0003-9861(87)90370-5. [DOI] [PubMed] [Google Scholar]
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Bruenger E., Rilling H. C. Determination of isopentenyl diphosphate and farnesyl diphosphate in tissue samples with a comment on secondary regulation of polyisoprenoid biosynthesis. Anal Biochem. 1988 Sep;173(2):321–327. doi: 10.1016/0003-2697(88)90196-0. [DOI] [PubMed] [Google Scholar]
- Chen Z., Morris C., Allen C. M. Changes in dehydrodolichyl diphosphate synthase during spermatogenesis in the rat. Arch Biochem Biophys. 1988 Oct;266(1):98–110. doi: 10.1016/0003-9861(88)90240-8. [DOI] [PubMed] [Google Scholar]
- Clarke C. F., Tanaka R. D., Svenson K., Wamsley M., Fogelman A. M., Edwards P. A. Molecular cloning and sequence of a cholesterol-repressible enzyme related to prenyltransferase in the isoprene biosynthetic pathway. Mol Cell Biol. 1987 Sep;7(9):3138–3146. doi: 10.1128/mcb.7.9.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick D. C., Carroll K. K. Extraction and quantitation of total cholesterol, dolichol and dolichyl phosphate from mammalian liver. Lipids. 1987 Dec;22(12):1045–1048. doi: 10.1007/BF02536448. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Squalene synthetase activity in human fibroblasts: regulation via the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5018–5022. doi: 10.1073/pnas.76.10.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki S., Nishino T., Katsuki H. Biosynthesis of isoprenoids in intact cells of Escherichia coli. J Biochem. 1986 Apr;99(4):1137–1146. doi: 10.1093/oxfordjournals.jbchem.a135577. [DOI] [PubMed] [Google Scholar]
- Fujisaki S., Nishino T., Katsuki H. Isoprenoid synthesis in Escherichia coli. Separation and partial purification of four enzymes involved in the synthesis. J Biochem. 1986 May;99(5):1327–1337. doi: 10.1093/oxfordjournals.jbchem.a135600. [DOI] [PubMed] [Google Scholar]
- Hiraishi A. High-performance liquid chromatographic analysis of demethylmenaquinone and menaquinone mixtures from bacteria. J Appl Bacteriol. 1988 Feb;64(2):103–105. doi: 10.1111/j.1365-2672.1988.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Gefter M., Mindich L. A mutant of Escherichia coli defective in DNA polymerase II activity. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3238–3242. doi: 10.1073/pnas.69.11.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDUTSCH A. A., PAULUS H., LEVIN E., BLOCH K. PURIFICATION OF GERANYLGERANYL PYROPHOSPHATE SYNTHETASE FROM MICROCOCCUS LYSODEIKTICUS. J Biol Chem. 1964 Aug;239:2507–2515. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Raetz C. R. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2274–2278. doi: 10.1073/pnas.72.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagami H., Ogura K. Geranylgeranyl pyrophosphate synthetase lacking geranyl-transferring activity from Micrococcus luteus. J Biochem. 1981 May;89(5):1573–1580. doi: 10.1093/oxfordjournals.jbchem.a133351. [DOI] [PubMed] [Google Scholar]
- Sagami H., Ogura K., Seto S., Kurokawa T. A new prenyltransferase from Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1978 Nov 29;85(2):572–578. doi: 10.1016/0006-291x(78)91201-9. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Ogura K. Farnesyl pyrophosphate synthetase from Bacillus subtilis. J Biochem. 1981 May;89(5):1581–1587. doi: 10.1093/oxfordjournals.jbchem.a133352. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Ogura K. Prenyltransferases of Bacillus subtilis: undecaprenyl pyrophosphate synthetase and geranylgeranyl pyrophosphate synthetase. J Biochem. 1982 Nov;92(5):1527–1537. doi: 10.1093/oxfordjournals.jbchem.a134077. [DOI] [PubMed] [Google Scholar]
- Umbreit J. N., Strominger J. L. Isolation of the lipid intermediate in peptidoglycan biosynthesis from Escherichia coli. J Bacteriol. 1972 Dec;112(3):1306–1309. doi: 10.1128/jb.112.3.1306-1309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



