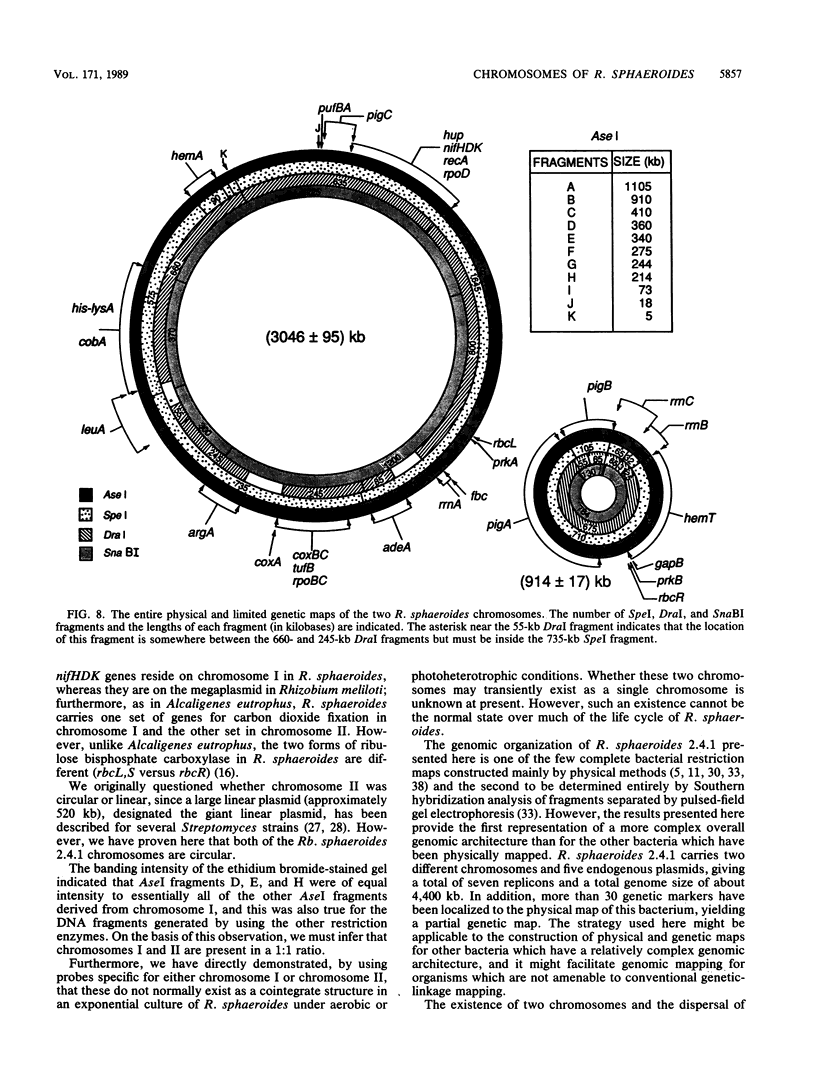
Abstract
A macrorestriction map representing the complete physical map of the Rhodobacter sphaeroides 2.4.1 chromosomes has been constructed by ordering the chromosomal DNA fragments from total genomic DNA digested with the restriction endonucleases AseI, SpeI, DraI, and SnaBI. Junction fragments and multiple restriction endonuclease digestions of the chromosomal DNAs derived from wild-type and various mutant strains, in conjunction with Southern hybridization analysis, have been used to order all of the chromosomal DNA fragments. Our results indicate that R. sphaeroides 2.4.1 carries two different circular chromosomes of 3,046 +/- 95 and 914 +/- 17 kilobases (kb). Both chromosome I (3,046 kb) and chromosome II (914 kb) contain rRNA cistrons. It appears that only a single copy of the rRNA genes is contained on chromosome I (rrnA) and that two copies are present on chromosome II (rrnB, rrnC). Additionally, genes for glyceraldehyde 3-phosphate dehydrogenase (gapB) and delta-aminolevulinic acid synthase (hemT) are found on chromosome II. In each instance, there appears to be a second copy of each of these genes on chromosome I, but the extent of the DNA homology is very low. Genes giving rise to enzymes involved in CO2 fixation and linked to the gene encoding the form I enzyme (i.e., the form I region) are on chromosome I, whereas those genes representing the form II region are on chromosome II. The complete physical and partial genetic maps for each chromosome are presented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfalvi Z., Kondorosi E., Kondorosi A. Rhizobium meliloti carries two megaplasmids. Plasmid. 1985 Mar;13(2):129–138. doi: 10.1016/0147-619x(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Bautsch W. Rapid physical mapping of the Mycoplasma mobile genome by two-dimensional field inversion gel electrophoresis techniques. Nucleic Acids Res. 1988 Dec 23;16(24):11461–11467. doi: 10.1093/nar/16.24.11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Malvick D. K., Mitchell R. E. Plasmid-mediated production of the phytotoxin coronatine in Pseudomonas syringae pv. tomato. J Bacteriol. 1989 Feb;171(2):807–812. doi: 10.1128/jb.171.2.807-812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Ely B., Gerardot C. J. Use of pulsed-field-gradient gel electrophoresis to construct a physical map of the Caulobacter crescentus genome. Gene. 1988 Sep 7;68(2):323–333. doi: 10.1016/0378-1119(88)90035-2. [DOI] [PubMed] [Google Scholar]
- Frank H. A., Taremi S. S., Knox J. R. Crystallization and preliminary X-ray and optical spectroscopic characterization of the photochemical reaction center from Rhodobacter sphaeroides strain 2.4.1. J Mol Biol. 1987 Nov 5;198(1):139–141. doi: 10.1016/0022-2836(87)90466-9. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Friedrich C. G., Meyer M., Schlegel H. G. Expression of hydrogenase in Alcaligenes spp. is altered by interspecific plasmid exchange. J Bacteriol. 1984 Apr;158(1):331–333. doi: 10.1128/jb.158.1.331-333.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Friedrich B. Regulation of hydrogenase formation is temperature sensitive and plasmid coded in Alcaligenes eutrophus. J Bacteriol. 1983 Jan;153(1):176–181. doi: 10.1128/jb.153.1.176-181.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower R. C., Bliska J. B., Cozzarelli N. R., Santi D. V. Analysis of amplified DNAs from drug-resistant Leishmania by orthogonal-field-alternation gel electrophoresis. The effect of size and topology on mobility. J Biol Chem. 1989 Feb 15;264(5):2979–2984. [PubMed] [Google Scholar]
- Hogrefe C., Friedrich B. Isolation and characterization of megaplasmid DNA from lithoautotrophic bacteria. Plasmid. 1984 Nov;12(3):161–169. doi: 10.1016/0147-619x(84)90040-4. [DOI] [PubMed] [Google Scholar]
- Hogrefe C., Römermann D., Friedrich B. Alcaligenes eutrophus hydrogenase genes (Hox). J Bacteriol. 1984 Apr;158(1):43–48. doi: 10.1128/jb.158.1.43-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979 Jan;2(1):1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Hynes M. F., Simon R., Pühler A. The development of plasmid-free strains of Agrobacterium tumefaciens by using incompatibility with a Rhizobium meliloti plasmid to eliminate pAtC58. Plasmid. 1985 Mar;13(2):99–105. doi: 10.1016/0147-619x(85)90062-9. [DOI] [PubMed] [Google Scholar]
- Kahn D., David M., Domergue O., Daveran M. L., Ghai J., Hirsch P. R., Batut J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J Bacteriol. 1989 Feb;171(2):929–939. doi: 10.1128/jb.171.2.929-939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi H., Shimaji M. Detection of giant linear plasmids in antibiotic producing strains of Streptomyces by the OFAGE technique. J Antibiot (Tokyo) 1987 Jun;40(6):913–916. doi: 10.7164/antibiotics.40.913. [DOI] [PubMed] [Google Scholar]
- Kinashi H., Shimaji M., Sakai A. Giant linear plasmids in Streptomyces which code for antibiotic biosynthesis genes. 1987 Jul 30-Aug 5Nature. 328(6129):454–456. doi: 10.1038/328454a0. [DOI] [PubMed] [Google Scholar]
- Klintworth R., Husemann M., Salnikow J., Bowien B. Chromosomal and plasmid locations for phosphoribulokinase genes in Alcaligenes eutrophus. J Bacteriol. 1985 Nov;164(2):954–956. doi: 10.1128/jb.164.2.954-956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Komiya H., Yeates T. O., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1: symmetry relations and sequence comparisons between different species. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9012–9016. doi: 10.1073/pnas.85.23.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O., Redfield R. J. Organization of the Haemophilus influenzae Rd genome. J Bacteriol. 1989 Jun;171(6):3016–3024. doi: 10.1128/jb.171.6.3016-3024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle L. E., Finch L. R. A physical map of the genome of Mycoplasma mycoides subspecies mycoides Y with some functional loci. Nucleic Acids Res. 1988 Jul 11;16(13):6027–6039. doi: 10.1093/nar/16.13.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata S., Higashitani A., Takanami M., Akiyama K., Kohara Y., Nishimura Y., Nishimura A., Yasuda S., Hirota Y. Construction of an ordered cosmid collection of the Escherichia coli K-12 W3110 chromosome. J Bacteriol. 1989 Feb;171(2):1214–1218. doi: 10.1128/jb.171.2.1214-1218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita F. R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988 Jun;52(2):155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]









