Abstract
Background
Since abnormalities in prostanoid metabolism occur in the lower airway of patients with cystic fibrosis (CF), it is likely that they could also be detected in the nose.
Methods
The degree of mRNA and protein expression of cyclo‐oxygenase (COX) enzymes 1 (COX‐1) and 2 (COX‐2) was examined using quantitative reverse competitive polymerase chain reaction (RT‐PCR) and Western blot analysis in the nasal polyps from 10 patients with CF, nasal polyps from 10 non‐CF patients and 11 nasal mucosa specimens. The results are presented as 106 cDNA molecules/μg total RNA and the densitometric ratio between protein and β‐actin.
Results
COX‐1 mRNA levels were significantly higher in CF nasal polyps (median 2.34, 25–75th percentiles 1.6–3.2) than in the nasal mucosa (0.78, 0.11–1.21), while there was no difference with non‐CF nasal polyps (1.11, 0.80–3.15). COX‐1 protein levels were significantly higher in CF nasal polyps (3.63, 2.71–4.27) than in nasal mucosa (1.55, 0.66–2.33) and non‐CF nasal polyps (2.19, 1.72–3.68). COX‐2 mRNA was significantly higher in CF nasal polyps (3.34, 2.42–7.05) than in nasal mucosa (1.69, 0.19–3.50). No differences were found in COX‐2 mRNA expression between CF and non‐CF polyps (1.38, 0.12–6.07). COX‐2 protein levels were also significantly higher in CF nasal polyps (0.23, 0.04–0.34) than in non‐CF nasal polyps (0.011, 0.009–0.016) or nasal mucosa (0.014, 0.014–0.016).
Conclusions
Upregulation in the expression of COX‐1 and COX‐2 could explain the high production of prostanoids reported in CF. These findings raise questions regarding the potential use of selective or non‐selective COX‐2 non‐steroidal anti‐inflammatory treatment in CF.
Keywords: cystic fibrosis, nasal polyp, cyclo‐oxygenase, COX‐1, COX‐2
Cystic fibrosis (CF) is an autosomal recessive disorder of exocrine glandular function. The basic metabolic derangement is related to a mutation in the gene regulating the CF transmembrane conductance regulator (CFTR) which acts as a chloride channel, regulating salt and water exchange in the apical membrane of epithelial cells. The genetic alteration results in excessive bronchial mucus secretion, reduced pancreatic enzyme secretion, and malabsorption in the gastrointestinal tract.1
Progressive lung disease characterised by chronic airway infection is the prime cause of morbidity and determines the prognosis and vital evolution.1 Chronic rhinosinusitis with or without nasal polyps also commonly occurs in CF.2,3 Because the defective mucus secretion affects exocrine glands along the entire respiratory tract, including the nose, it is generally accepted that this finding contributes to the high incidence of chronic rhinosinusitis in patients with CF.1
The traditional hypothesis postulates that pro‐inflammatory substances produced by various cells stimulated by infectious agents are responsible for CF lung disease.1 However, recent studies have suggested that inflammation may even occur in the absence of infection. An increase in markers of inflammation has been found in the lungs of patients with mild disease, as well as in those in stable clinical conditions.4,5,6,7
In response to infectious agents, high levels of interleukin 8 (IL‐8), 1 (IL‐1), 6 (IL‐6) and tumour necrosis factor‐α (TNF‐α) have been detected in CF sputum and bronchoalveolar lavage (BAL) fluid.8,9 Since the intensity of the inflammatory reaction appears to be, at least in part, independent of the infectious stimulus, it has been suggested that early and excessive inflammation may be related to constitutive abnormalities associated with a defective CFTR or otherwise.4,7,10,11,12
In addition to increased release of pro‐inflammatory cytokines, several studies have also demonstrated overproduction of leukotrienes13,14 and prostaglandins E2 and F2α.15,16,17 Prostaglandin endoperoxide synthase, commonly called cyclo‐oxygenase (COX), is the key enzyme required for the conversion of arachidonic acid into prostaglandins (PGs). Two COX isoforms have been identified: COX‐1 and COX‐2. COX‐1 is constitutively expressed in most tissues where it maintains the physiological processes.18 COX‐2 is highly inducible at inflammatory sites and is considered the main target for anti‐inflammatory therapies.18,19 Ibuprofen, a non‐steroidal anti‐inflammatory drug (NSAID) that works by inhibiting both COX enzymes, has been shown to be an effective drug in patients with CF. High‐dose ibuprofen slowed the decline in lung function in patients with CF, the benefit being most evident in patients between 5 and 13 years of age.20
Because there is an overproduction of PGs in CF, and because PG production under conditions of inflammation is dependent on the induction of COX‐2, we hypothesised that an upregulated COX‐2 enzyme should account for the increased production of prostanoids. The purpose of this study was to examine the degree of expression of COX‐1 and COX‐2 in the nose of patients with CF. This study was designed as the first step in the assessment of the potential therapeutic effects of selective COX‐2 inhibitors in CF patients.
Methods
Subjects
Endoscopy sinus surgery and polypectomy was offered to 10 CF patients with nasal polyps filling up the nasal cavity and without any substantial clinical and endoscopic response after at least 1 year of intranasal glucocorticoid therapy and short courses of oral glucocorticoid. Glucocorticoid treatment was discontinued at least 2 weeks before surgery. A definitive diagnosis of CF was made on the basis of raised sweat electrolytes (Cl− level >60 mmol/l) and identification of associated CFTR mutations.
Nasal polyps were obtained from 10 aspirin tolerant patients undergoing polypectomy. Nasal mucosa was also obtained from 11 patients undergoing nasal corrective surgery (healthy control group). The 10 patients with nasal polyps had not received glucocorticoids for at least 2 weeks before surgery. None of the healthy subjects and non‐CF patients had suffered from upper respiratory infection during the 4 weeks before surgery, nor had any of the CF patients suffered an acute upper airway infective exacerbation of their chronic process within the 4 weeks before polypectomy.
The subjects or their parents agreed to participate in the study, which was approved by the ethics committees of our institutions.
Atopy
Atopic status was assessed by skin prick testing to a panel of common aeroallergens present in the local geographical area (Bial Aristegui Laboratories, Bilbao, Spain) according to standard methods. Patients were considered atopic if they had a positive reaction to at least one of the allergens tested.
Microscopic analysis
Surgical specimens were examined using standard haematoxylin‐eosin staining methods by a pathologist who was blind to the clinical data. Inflammatory cells, including eosinophils, lymphocytes, plasma cells and polymorphonuclear cells, were quantified and the results were related to 100 inflammatory cells.
Reverse transcriptase (RT)‐competitive polymerase chain reaction (PCR)
RNA extraction and reverse transcription
A specimen obtained at the time of surgery was immediately snap frozen in liquid nitrogen and kept at −80°C until analysed. Total RNA from nasal tissue specimens was isolated using a rapid extraction method (TRI‐Reagent), as previously described.21 Total RNA (4 μg) from nasal mucosa and nasal polyps was reverse transcribed to cDNA using random hexanucleotide primers and SuperScript II Rnase reverse transcriptase (Invitrogem, Paisley, UK).
Primer design
COX‐1 and COX‐2 primers were designed to span introns using the following sequences: COX‐1 sense (nucleotides 516–539): 5′ TGCCCAGCTCCTGGCCCGCCGCTT 3′; COX‐1 antisense (nucleotides 769–819): 5′ GTGCATCAACACAGGCGCCTCTTC 3′; COX‐2 sense (nucleotides 574–600): 5′ TTCAAATGAGATTGTGGGAAAATTGCT 3′; COX‐2 antisense (nucleotides 855–878): 5′ AGATCATCTCTGCCTGAGTATCTT 3′.
Competitive polymerase chain reaction
To measure COX‐1 and COX‐2 mRNA expression we developed a previously reported competitive PCR technique in which, after the reverse transcription step, known amounts of an exogenous DNA (external standard) were added to the amplification mixture (RT‐competitive PCR). The exogenous molecule (called a competitor) was co‐amplified in competition with the target in the same test tube. Since the initial amount of internal standard was known, this technique permitted the determination of the initial amount of target cDNA. The reverse transcription and PCR reaction conditions have previously been reported.21
Western blot analysis
Frozen nasal samples were placed in cold sterile tubes with 200 μl lysate buffer (a complete protease inhibitor cocktail tablet in 50 ml 0.05 M Hepes buffer solution, 0.05% v/v Triton X‐100, and 625 μM PMSF). Tissue samples were sonicated twice for 15 seconds in a sonifier (Branson; Danbury, CT, USA) and centrifuged at 12 000g for 10 minutes at 4°C. The supernatants were removed and kept at −80°C for later use. Total proteins were measured using a modified Lowry method in which the absorbencies were read at 630 nm in a microplate reader (Biotek Instruments; Winooski, VT, USA). Total protein concentrations were interpolated from a standard curve prepared with bovine serum albumin using the Delta soft ΙΙ 4.0 software package. The assay range was 50–400 μg/ml. Ten μl (50 μg) of total protein was added to 5 μl loading buffer (NuPAGE LDS sample buffer) and spun down. Samples were then heated in a thermocycler to 70°C for 10 minutes. Rainbow molecular weight marker (15 μl, Amersham) or samples were loaded in 7% TRIS acetate gels and run (125 V, 90 minutes) in a Novex XCell ΙΙ Mini‐Cell (San Diego, CA, USA). Following electrophoresis, proteins were transferred (30 V, 1 hour) to a 0.45 μm pore size nitrocellulose membrane using a Novex XCell ΙΙ Blot Module. To check for equal loading and transfer efficiency, membranes were stained with Ponceau S (0.5% in 1% acetic acid). Membrane non‐specific binding sites were blocked using blocking buffer (5% non‐fat dry milk, 0.1 % Tween 20 in 10 nM phosphate buffered saline (PBS)) for 1 hour at room temperature in an orbital shaker. The membranes were incubated with goat polyclonal IgG anti‐COX‐1 or anti‐COX‐2 antibodies in blocking buffer (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), then washed four times in 0.5% Tween 20 in 10 nM PBS. The membranes were then incubated with peroxidase conjugated anti‐goat IgG in blocking buffer (1:3000). After a new series of washes, immunoreactive bands were visualised using light emitting chemoluminescence (Supersignal West Pico Chemiluminescent Substrate) and the light emissions were detected by CCD Camera System LAS 3000 (Fujufilm). The intensities of the bands were quantified with Fujifilm Image Gauge 4.0 Software. The membranes were re‐probed with a mouse anti‐human β‐actin monoclonal antibody (Sigma) at a dilution of 1:7500. The intensities of the bands were quantified and used as a loading control.
Statistical analysis
The results are presented as medians with 25–75th percentiles and as mean (SD) values. Non‐parametric statistical analysis was performed using the Kruskal‐Wallis and Mann‐Whitney U tests. A p value of <0.05 was considered statistically significant.
Results
Subjects
The demographic characteristics of the three groups are shown in table 1. CF patients were significantly younger than non‐CF subjects.
Table 1 Demographic characteristics of study subjects.
| Group | N | Age (years) | Sex (M/F) | Atopy (yes/no) |
|---|---|---|---|---|
| Nasal mucosa | 11 | 32 (1.9), (18–54) | 8/3 | 2/9 |
| Non‐CF nasal polyps | 10 | 51 (4), (40–63) | 7/3 | 3/7 |
| CF nasal polyps | 10 | 14.5 (7), (7–31)* | 7/3 | 3/7 |
Age is expressed as mean (SD) and range.
*p<0.05 v CF nasal polyps and nasal mucosa.
Histological analysis
The histological study showed a higher percentage of eosinophils in non‐CF nasal polyps (29%, p<0.05) than in nasal mucosa (3%) or CF nasal polyps (5%). There were no significant differences in the number of lymphocytes, polymorphonuclear cells, and plasma cells in the different tissues (table 2).
Table 2 Results of histological analysis.
| Tissue | Lymphocytes (%) | Plasma cells (%) | Polymorphonuclear cells (%) | Eosinophils (%) |
|---|---|---|---|---|
| Nasal mucosa | 42 (32.5–40) | 50 (47.5–55) | 5 (4–5) | 3 (2–9) |
| Non‐CF nasal polyps | 35 (15–45) | 32 (25–40) | 4 (4–10) | 29 (10–50)* |
| CF nasal polyps | 34 (20–36) | 52 (49–61) | 9 (5–16) | 5 (3–10) |
Results are expressed as median (25–75th percentile).
*p<0.05 v nasal mucosa and CF nasal polyps.
COX‐1 mRNA and protein
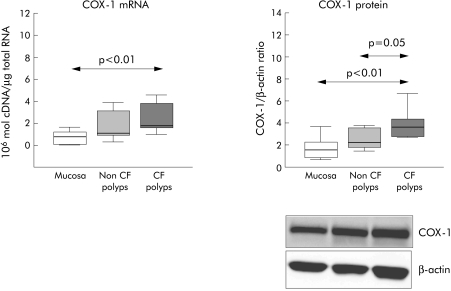
The mean levels of COX‐1 mRNA expression in nasal polyps from CF patients were significantly higher (p<0.01) than in nasal mucosa. There was no difference in COX‐1 mRNA levels between CF and non‐CF nasal polyps and between non‐CF nasal polyps and nasal mucosa (fig 1). Upregulation of mRNA in CF nasal polyps was also found in COX‐1 protein levels; Western blot analysis showed statistically significant higher levels in CF nasal polyps than in non‐CF nasal polyps (p = 0.05) and nasal mucosa (p<0.01, fig 1).
Figure 1 RT‐PCR (106 cDNA molecules/μg total RNA) and Western blot analysis (densitometric ratio between protein and β‐actin) of COX‐1 in CF nasal polyps, non‐CF nasal polyps, and nasal mucosa. Box plots show the 25th, 50th (median), and 75th percentile values. Whiskers show the minimum and maximum values. CF, cystic fibrosis.
COX‐2 mRNA and protein
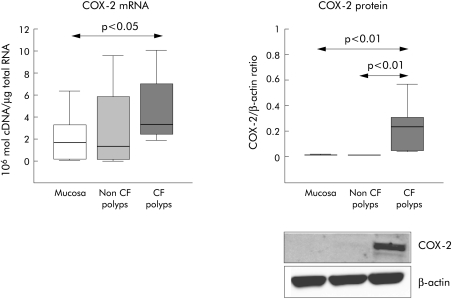
The mean levels of COX‐2 mRNA expression in nasal polyps from CF patients were significantly higher (p<0.05) than in nasal mucosa from healthy subjects. No differences were found between nasal mucosa and non‐CF nasal polyps (fig 2). COX‐2 protein was detected in CF nasal polyps but it was almost undetectable in nasal mucosa and non‐CF nasal polyps (fig 2).
Figure 2 RT‐PCR (106 cDNA molecules/μg total RNA) and Western blot analysis (densitometric ratio between protein and β‐actin) of COX‐2 in CF nasal polyps, non‐CF nasal polyps, and nasal mucosa. Box plots show the 25th, 50th (median), and 75th percentile values. Whiskers show the minimum and maximum values. CF, cystic fibrosis.
Discussion
Previous studies have reported high levels of prostanoids in the BAL fluid, saliva, and urine of patients with CF.15,16,17 On the basis of these findings, we hypothesised that high levels of prostanoids should result from upregulated COX‐2, the enzyme responsible for the increased production of prostanoids under conditions of inflammation.18 Because there is compelling evidence to support the notion that nasal respiratory mucosa shares the histological characteristics and ion transport abnormalities of lower airways mucosa in patients with CF,22 we used nasal respiratory samples to test our hypothesis.
Our study shows that CF nasal polyps are characterised by a marked activation of COX‐1 and COX‐2, which was reflected in the high expression of both mRNA and protein. The increased expression of COX‐2 detected in our study concurs with the generally accepted theory that predicts an upregulation of this enzyme in inflamed tissues.18,19 However, it is interesting to note that previous studies have reported an abnormal downregulation of COX‐2 mRNA in nasal polyps originating in patients with inflammatory diseases other than CF.21,22,23,24,25,26,27,28,29 Accordingly, COX‐2 protein is not usually found or is detected only in low amounts by Western blot analysis in both healthy tissues and in non‐CF nasal polyps.21,24 These abnormalities in COX‐2 regulation are associated with a reduced production of prostaglandin E2 in polyps from aspirin intolerant patients.23,29
In a previous study we reported a significantly lower expression of COX‐2 mRNA in nasal polyps than in nasal mucosa.26 We also found that these differences were detectable only when nasal samples were frozen immediately after surgery. We could not replicate these findings in the present study, probably because the nasal samples were collected from various hospitals some distance away, and the protocol for handling specimens could not be followed strictly.
The mechanism involved in the different regulation of COX‐2 in nasal polyps obtained from non‐CF and CF patients is as yet unclear. Although CF patients were significantly younger than non‐CF subjects, differences in age cannot explain the marked differences in the level of expression of COX‐2 protein found in our study. In fact, COX‐2 was undetectable in all subjects from the two non‐CF groups, including several patients of similar age to CF patients.
The detection of high levels of COX‐2 protein in CF polyps supports the notion that the increased levels of prostanoids in urine, BAL fluid, and sputum reported in CF patients are the consequence of marked activation of the COX‐2 enzyme. The increased expression of COX‐1 mRNA and protein in CF nasal polyps suggests that this enzyme may also contribute to the increased production of prostanoids in patients with CF. This was an unexpected finding because COX‐1 is usually considered an enzyme with merely physiological roles that is not involved in inflammatory responses. However, this is not always the case since upregulation of COX‐1 has been reported in some inflammatory situations such as in the gastric mucosa.30
Our observations raise important questions regarding the origin of the reported benefits of drugs such as ibuprofen, which inhibit the two COX enzymes, in the treatment of CF. Is the efficacy of ibuprofen related to its capacity to inhibit both COX‐1 and COX‐2? Would a selective COX‐2 inhibitor be more or less effective?
It is generally accepted that the main physiological regulator of CFTR is cAMP. Prostanoids such as PGE2 stimulate the increase of this intracellular signal and thereby activate CFTR and increase chloride efflux. Recent studies have shown that pro‐inflammatory cytokines such as IL‐1β can reduce cAMP accumulation and chloride efflux in response to PGE2. This effect appears to be mediated by an autocrine loop involving induction of COX‐2 and endogenous PGE2 production. It is likely that, by breaking this loop using COX‐2 inhibitors, the deleterious effects of inflammation on PGE2 and on chloride efflux can be reduced.31
Advances in research on NSAIDs have permitted the development of a new class of drugs with selective COX‐2 inhibitory effects.19 Whether selective COX‐2 inhibitors could be more effective and better tolerated by patients with CF than unselective COX inhibitors is something that needs to be tested in a comparative study of the two types of drugs. However, the use of selective COX‐2 inhibitors as anti‐inflammatory drugs in CF and other chronic inflammatory diseases has recently been questioned because of the finding of a significant increase in the cardiovascular risk in patients on long term treatment with these new drugs.32
The role of prostanoids in CF is far from clear. The traditional view is that prostanoids have a negative and pro‐inflammatory role in some inflammatory diseases, which justifies the need to limit their production through the use of NSAIDs. However, recently reported findings suggest that the role of prostanoids is much more complex, to the point that they may also act by controlling inflammation in some chronic lung inflammatory processes.33 Interestingly enough, these potential salutary effects of prostanoids have been demonstrated in CF. The administration of misoprostol, a synthetic prostaglandin E1 analogue, improves fat malabsorption in CF—an observation which suggests that the inhibition of prostanoid synthesis may not always be beneficial to patients with CF.34
In summary, our study shows upregulation in the expression of COX‐1 and COX‐2 in the nasal polyps of CF patients. This finding could explain the high production of prostanoids reported in this disease and detected in BAL fluid, saliva, and urine. Our results also raise questions regarding the potential use of selective or non‐selective COX‐2 NSAIDs in CF.
Abbreviations
BAL - bronchoalveolar lavage
CF - cystic fibrosis
IL - interleukin
NSAID - non‐steroidal anti‐inflammatory drug
PG - prostaglandin
TNF‐α - tumour necrosis factor α
Footnotes
This study was supported by grants from Fondo de Investigaciones Sanitarias (FIS 00‐0802), La Marató TV3 and Ministerio de Educación y Ciencia (SAF‐2002‐04431‐C02‐01) Red RESPIRA (Fis, V‐2003‐REDC11D‐0).
Competing interests: none declared.
References
- 1.Davis P B, Druman M, Konstan M W. Cystic fibrosis: state of the art. Am J Respir Crit Care Med 19961541229–1256. [DOI] [PubMed] [Google Scholar]
- 2.Isaacson G, Yanagisawa E. Cystic fibrosis and sinusitis. Ear Nose Throat J 199877886–888. [PubMed] [Google Scholar]
- 3.Hadfield P J, Rowe‐Jones J M, Mackay I S. The prevalence of nasal polyps in adults with cystic fibrosis. Clin Otolaryngol 20002519–22. [DOI] [PubMed] [Google Scholar]
- 4.Konstan M W, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol 199724137–142. [DOI] [PubMed] [Google Scholar]
- 5.Kahn T Z, Wagener J S, Bost T.et al Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995111075–1082. [DOI] [PubMed] [Google Scholar]
- 6.Amstrong D S, Grimwood K, Carzino R.et al Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 19953101571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstan M W, Hilliard K A, Norvell T M.et al Bronchoalveolar lavage findings in cystic fibrosis patients with stable clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med 1994150448–454. [DOI] [PubMed] [Google Scholar]
- 8.Dean T P, Dai Y, Shute J K.et al Interleukin‐8 concentrations are elevated in bronchoalveolar lavage, sputum and sera of children with cystic fibrosis. Pediatr Res 199334159–161. [DOI] [PubMed] [Google Scholar]
- 9.Bonfield T L, Panuska J R, Konstan M W.et al Inflammatory cytokines in cystic fibrosis lung. Am J Respir Crit Care Med 199 1522111–2118. [DOI] [PubMed] [Google Scholar]
- 10.Wagener J S, Kahn T Z, Copenhaver S C.et al Early inflammation and the development of pulmonary disease in cystic fibrosis. Pediatr Pulmunol 199716267–268. [DOI] [PubMed] [Google Scholar]
- 11.Muhlebach M S, Stewart P W, Leigh M W.et al Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 1999160186–191. [DOI] [PubMed] [Google Scholar]
- 12.Muhlebach M S, Noah T L. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am J Respir Crit Care Med 2002165911–915. [DOI] [PubMed] [Google Scholar]
- 13.Konstan M W, Walenga R W, Hilliard K A.et al Leukotriene B4 markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am Rev Respir Dis 1993148896–901. [DOI] [PubMed] [Google Scholar]
- 14.Sampson A P, Spencer D A, Green C P.et al Leukotrienes in sputum and urine of cystic fibrosis children. Br J Clin Pharmacol 199030861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemen R J, Gates A J, Mathé A A.et al Relationships among digital clubbing, disease severity, and sputum prostaglandin F2α an E concentrations in cystic fibrosis patients. Am Rev Respir Dis 1978117639–646. [DOI] [PubMed] [Google Scholar]
- 16.Strandvik B, Svensson E, Seyberth H W. Prostanoid biosynthesis in patients with cystic fibrosis. Prostagland Leukot Essent Fatty Acids 199655419–425. [DOI] [PubMed] [Google Scholar]
- 17.Rigas B, Korenberg J R, Merill W W.et al Prostaglandins E2 and F2 alpha are elevated in saliva of cystic fibrosis patients. Am J Gastroenterol 1989841408–1412. [PubMed] [Google Scholar]
- 18.Smith W L, DeWit D L, Garavito R M. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 200069145–182. [DOI] [PubMed] [Google Scholar]
- 19.Turine M E, DuBois R N. Cyclooxygenase‐2: a therapeutic target. Annu Rev Med 20025335–57. [DOI] [PubMed] [Google Scholar]
- 20.Konstan M W, Byard P J, Hoppel C H.et al Effect of high‐dose ibuprofen in patients with cystic fibrosis. N Engl J Med 1995332848–854. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez‐Morata J C, Mullol J, Fuentes M.et al regulation of cyclooxygenase‐1 and‐2 in human nasal mucosa. Effects of cytokines and dexamethasone. Clin Exp Allergy 2000301275–1284. [DOI] [PubMed] [Google Scholar]
- 22.Knowles M R, Gatzy J, Boucher R C. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med 19813051489–1495. [DOI] [PubMed] [Google Scholar]
- 23.Picado C, Fernandez‐Morata F, Juan M.et al Cycloxygenase‐2 mRNA is downexpressed in nasal polyps from aspirin‐sensitive asthmatics. Am J Respir Crit Care Med 1999160291–296. [DOI] [PubMed] [Google Scholar]
- 24.Mullol J, Fernandez‐Morata J C, Roca‐Ferrer J.et al Cyclooxygenase 1 and cyclooxygenase 2 expression is abnormally regulated in human nasal polyps. J Allergy Clin Immunol 2002109824–830. [DOI] [PubMed] [Google Scholar]
- 25.Picado C, Bioque G, Roca‐Ferrer J.et al Nuclear factor‐κB is down‐regulated in nasal polyps from aspirin‐sensitive asthmatics. Allergy 200258122–126. [DOI] [PubMed] [Google Scholar]
- 26.Pujols L, Mullol J, Alobid I.et al Dynamics of COX‐2 in nasal mucosa and nasal polyps from aspirin‐tolerant and aspirin‐intolerant patients with asthma. J Allergy Clin Immunol 2004114814–819. [DOI] [PubMed] [Google Scholar]
- 27.Perez‐Novo C A, Watgelet J B, Claeys C.et al Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol 20051151189–1196. [DOI] [PubMed] [Google Scholar]
- 28.Schmid D, Gode U, Shäfer D.et al Arachidonic acid metabolism in nasal tissue and peripheral blood cells in aspirin intolerant asthmatics. Acta Otolaryngol 1999119277–280. [DOI] [PubMed] [Google Scholar]
- 29.Kowalski M L, Pawliczak R, Wozniak J.et al Differential metabolism of arachidonic acid in nasal polyp epithelial cells cultured from aspirin‐sensitive and aspirin‐tolerant patients. Am J Respir Crit Care Med 2000161391–398. [DOI] [PubMed] [Google Scholar]
- 30.Wallace J L, McNight W, Reuter B K.et al NSAID‐induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 2000119706–714. [DOI] [PubMed] [Google Scholar]
- 31.Clayton A, Holland E, Pang L.et al Interleukin‐1β differentially regulates β2 adrenoreceptor and prostaglandin E2‐mediated cAMP accumulation and chloride efflux from Calu‐3 bronchial epithelial cells. J Biol Chem 200528023451–23463. [DOI] [PubMed] [Google Scholar]
- 32.FitzGerald G A. Coxibs and cardiovascular disease. N Engl J Med 20043511709–1711. [DOI] [PubMed] [Google Scholar]
- 33.Gilroy D W, Colville‐Nash P R, Willis D.et al Inducible cyclooxygenase may have antiinflammatory properties. Nat Med 19995698–701. [DOI] [PubMed] [Google Scholar]
- 34.Robinson P J, Sly P D, Smith A L. Effect of misoprostol on fat malabsorption in cystic fibrosis. Arch Dis Child 1988631081–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]