Abstract
Background
Bronchial hyperresponsiveness (BHR) is a common feature of asthma. However, BHR is also present in asymptomatic individuals and its clinical and prognostic significance is unclear. We hypothesised that BHR might play a role in the development of chronic obstructive pulmonary disease (COPD) as well as asthma.
Methods
In 1991 respiratory symptoms and BHR to methacholine were evaluated in 7126 of the 9651 participants in the SAPALDIA cohort study. Eleven years later 5825 of these participants were re‐evaluated, of whom 4852 performed spirometric tests. COPD was defined as an FEV1/FVC ratio of <0.70.
Results
In 1991 17% of participants had BHR, of whom 51% were asymptomatic. Eleven years later the prevalence of asthma, wheeze, and shortness of breath in formerly asymptomatic subjects with or without BHR was, respectively, 5.7% v 2.0%, 8.3% v 3.4%, and 19.1% v 11.9% (all p<0.001). Similar differences were observed for chronic cough (5.9% v 2.3%; p = 0.002) and COPD (37.9% v 14.3%; p<0.001). BHR conferred an adjusted odds ratio (OR) of 2.9 (95% CI 1.8 to 4.5) for wheezing at follow up among asymptomatic participants. The adjusted OR for COPD was 4.5 (95% CI 3.3 to 6.0). Silent BHR was associated with a significantly accelerated decline in FEV1 by 12 (5–18), 11 (5–16), and 4 (2–8) ml/year in current smokers, former smokers and never smokers, respectively, at SAPALDIA 2.
Conclusions
BHR is a risk factor for an accelerated decline in FEV1 and the development of asthma and COPD, irrespective of atopic status. Current smokers with BHR have a particularly high loss of FEV1.
Keywords: bronchial hyperresponsiveness, asthma, chronic obstructive pulmonary disease, smoking, epidemiological study
Bronchial hyperresponsiveness (BHR) is a common finding in asthma1 and has also been observed in patients with chronic obstructive airways disease (COPD).2 Cross sectional studies have found significant associations between BHR and respiratory symptoms, including wheezing, cough and shortness of breath.3,4,5 Population based studies, including the first cross sectional Swiss study on Air Pollution and Lung Diseases in Adults (SAPALDIA), suggest that 11–20% of individuals have BHR.4,6 However, a significant proportion of individuals with BHR do not suffer from respiratory symptoms, asthma or other obstructive airways diseases. It is thought that the proportion of asymptomatic individuals with BHR ranges from 19% to 62% in the general population.7 Thus, many subjects with BHR are asymptomatic or “silent”. Although the presence of BHR has been positively associated with the development of respiratory symptoms and negatively associated with symptom remission in a longitudinal study of 2684 adults,8,9 the relevance and long term impact of BHR in the absence of symptoms has not been fully elucidated.
Prevailing current opinion is that classical asthma is characterised by two main features that occur together: (allergic) inflammation with airway thickening and mucus formation,10 and airway smooth muscle dysfunction with BHR.11 BHR or airway inflammation alone are probably not sufficient to cause asthma, but might be independent risk factors for the development of symptomatic airway dysfunction.12 Indeed, there is good evidence that allergic sensitisation is a risk factor for the development of asthma.13,14 In a relatively small study of 194 adults, Segala et al15 found that BHR to methacholine was a predictor of wheezing in a 5 year follow up study, independent of atopic status. In asthmatics, prolonged treatment with inhaled or systemic corticosteroids can reduce BHR but often does not abrogate it.16 Thus, although often associated with airway inflammation,17 it has not yet clear whether BHR is truly an independent risk factor for the development of asthma.
Even less is known about the association between BHR and COPD. The probability of a decline of 20% or more in FEV1 in response to a provoking concentration of <3000 μmol inhaled methacholine is partially dependent on baseline lung function,18 which must be addressed in studying the relation between BHR and COPD. However, apart from its role in the modern dual feature asthma hypothesis, BHR could also be a risk factor for the development and progression of COPD, particularly in situations where BHR occurs alongside a non‐allergic, typically cigarette smoke induced airway inflammation. The potential interaction between smoking and BHR was documented in the Lung Health Study,19 a 5 year randomised prospective clinical study of 4201 patients with mild COPD. The study showed that BHR improved after smoking cessation. Similarly, the European Community Respiratory Health Survey,20 a large epidemiological study of random population samples of 22 European regions, found that smoking was a risk factor for an increase in BHR over time in 3993 participants. On the other hand, the Normative Aging Study,21 which studied 435 men and excluded those with symptoms, found no relation between change in BHR over 3 years and smoking status. In a study of bronchial biopsy specimens, Willemse et al22 found that bronchial inflammation was similar in current smokers with COPD and zasymptomatic smokers but lower in non‐smoking patients with COPD. The authors concluded that the inflammatory effects of current smoking may mask the underlying ongoing inflammatory process pertinent to COPD. Thus, smoking could play the role of a non‐specific “amplifier”.
The current population based longitudinal survey investigates the relevance of BHR in asymptomatic adults with respect to a range of prospective clinical outcomes. We hypothesised that asymptomatic BHR in 1991 is a risk factor for the development of respiratory symptoms, and a risk factor for asthma and COPD 11 years later.
Methods
Study design and population
The methodology and selection of the participants of the SAPALDIA prospective cohort study have been described in detail elsewhere.23,24 The population was a random sample (18–60 years) recruited from eight areas of Switzerland using population registries in 1990. Health examinations were conducted for SAPALDIA 1 in 1991. The second round of health examinations in 2002 (SAPALDIA 2) included identical protocols to those in the first survey. Of the 9651 participants in 1991, 7126 had a methacholine challenge. Subjects were included in the present analysis if spirometric and bronchial challenge data were available from SAPALDIA 1 and questionnaire data were available from both surveys (n = 5825). Of these, 4852 performed spirometric tests at both surveys.
Ethical approval for the study was given by the central ethics committee of the Swiss Academy of Medical Sciences and the Cantonal ethics committees for each of the eight examination centres.
Respiratory symptoms, phenotype definitions, and smoking habits
Information about respiratory symptoms, smoking habits, and other risk factors was gathered through an interview administered questionnaire based on the European Community Respiratory Health Survey (ECRHS) questionnaire.25 Symptoms examined were “wheeze without cold in the last 12 months”, “shortness of breath when hurrying on level ground or walking up a slight hill”, “chronic cough—cough during the day or night on most days for as much as 3 months each year for more than 2 years”, and “chronic phlegm—phlegm during the day or night on most days for as much as 3 months each year for more than 2 years”. Asymptomatics were defined as participants without wheeze, shortness of breath, chronic cough, chronic phlegm, or physician‐diagnosed asthma at SAPALDIA 1. Participants with a forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio of <0.70 without a physician's diagnosis of asthma were classified as having evidence of COPD.26 Asthma was defined as physician‐diagnosed asthma. Smokers were participants who had smoked ⩾20 packs of cigarettes or ⩾360 g of tobacco in their lifetime. Former smokers were smokers who had quit smoking at least 1 month before examination in 2002 and current smokers were participants who reported active smoking at the interview in 2002. The cigarette exposure of participants was assessed by pack‐years. Participants were asked not to smoke in the hour before the examination and recent exposure to smoking was validated by the measurement of carbon monoxide (CO) concentration in exhaled air using an EC50 Micro‐Smokerlizer.
Assessment of pulmonary function, bronchial responsiveness, and atopy
The same spirometers (SensorMedics 2200 SP Yorba Linda, USA) were used in 1991 and in 2002.24,27 The protocol for the measurement of lung volumes and flows was identical to that in the ECRHS and complied with American Thoracic Society recommendations.5 Participants were requested not to use short acting inhalers 4 hours and long acting inhalers 8 hours before the examination appointment.
Participants able to produce technically satisfactory spirometric values and who satisfied health inclusion criteria were invited to undergo a bronchial challenge. Non‐specific bronchial responsiveness was assessed by bronchial challenge with methacholine chloride administered by MEFAR aerosol dosimeters.23 The challenge schedule started with an inhalation of saline followed by increasing concentrations of methacholine up to a cumulative dose of 8.37 μmol. The test was stopped when either the maximum cumulative dose had been reached or FEV1 had fallen by 20% or more.6 BHR to methacholine was defined as a fall of 20% or more in FEV1 compared with the highest FEV1 value measured during the test in response to inhalation of methacholine to the maximum dose, and the degree of bronchial reactivity was measured by calculating a dose‐response slope.28 The slope was defined as the ratio between the percentage decline in FEV1 and the total cumulative dose administered. Since the distribution of the slopes was skewed, data were transformed using natural logarithms before analysis. A small constant (0.01) was added before transformation in order not to lose observations with zero slope.
Skin prick tests were conducted in 1991 in accordance with the ECRHS allergy testing protocol and included testing sensitisation to house dust mite (Dermatophagoides pteronyssinus), cat, dog, fungi (Cladosporium and Alternaria spp), timothy grass, birch and parietaria pollen.23,25 Participants were classified as atopic if they developed a skin wheal to one or more of the allergens with a mean diameter exceeding the negative control wheal by at least 3 mm.
Statistical analysis
Univariate and bivariate analyses were conducted initially to provide descriptive statistics. Logistic regression was used to model relations between the presence and absence of BHR in asymptomatic subjects in 1991 and new symptoms at follow up in 2002 while adjusting for potential confounders. Factors tested as potential confounders included age, sex, atopy, smoking status, pack years, FVC at baseline, height, body mass index at baseline, change in weight, level of education, exposure to environmental tobacco smoke (ETS), exposure to dust and fumes at work and study area. FVC was included as a proxy for lung size and airway calibre.6,29 In addition, effect modification of the relation between BHR and new symptoms by sex, atopy, and smoking status (current, former or never smoker at SAPALDIA 2) were investigated. The relation between responsiveness to methacholine and symptoms and COPD 11 years later was also examined with responsiveness measured by the continuous variable “slope”. Potential non‐linearities in the associations between methacholine slope at SAPALDIA 1 and outcomes at SAPALDIA 2 were tested by adding the square and the cube of slope as covariates. Percentage risks of new symptoms or COPD at follow up associated with the presence or absence of BHR were estimated from the logistic regression models upon adjusting covariates to their population means.
The effect of BHR at baseline on change in FEV1 was modelled by linear regression adjusting for the relevant confounders listed above and for baseline FEV1. Analyses were conducted using Stata Special Edition release 8.2 (Stata Corporation, Texas, USA). p values of <0.05 and <0.1 were interpreted as statistically significant for main and interaction effects, respectively.
Results
Population characteristics
Of the initial 9651 participants in SAPALDIA 1, 7126 underwent a methacholine challenge. Reasons for lack of a challenge included technically poor baseline spirometry, refusal, exclusions on the basis of health criteria including heart disease, epilepsy, pregnancy, lactation, and use of β blockers,23 and 135 (1.4%) participants were excluded because of a baseline FEV1 <70% predicted or <1.5 l.30 Of the 7126 participants with a valid bronchial challenge test in 1991, 5825 were re‐evaluated in 2002 and are included in the current analyses. Of these, 4852 performed spirometric tests and 3931 were asymptomatic at SAPALDIA 1. A total of 222 participants were excluded from the multivariate analyses because of inconsistent information about smoking habits between surveys or exhaled carbon monoxide concentrations of more than 10 ppb, despite claiming to be a never or former smoker.
Non‐participants in the second evaluation in 2002 (n = 1301) were compared with the participants in both evaluations (table 1). Slightly more men, smokers, persons with low educational background, with professional exposure to fumes and dust, and more individuals with respiratory symptoms were non‐participants in the follow up evaluation.
Table 1 Characteristics of subjects with bronchial challenge at baseline according to whether they participated in both surveys or only in the first one.
| SAPALDIA 1 & 2 (n = 5825) | SAPALDIA 1 only (n = 1301) | p value | |
|---|---|---|---|
| Women (% ) | 50.3 | 45.5 | 0.002 |
| Mean (SD) height (cm) | 169 (9) | 169 (9) | 0.14 |
| Mean (SD) weight (kg) | 69 (13) | 69 (14) | 0.12 |
| Mean (SD) FEV1 (l/s) | 3.6 (0.8) | 3.6 (0.8) | 0.378 |
| Mean (SD) FVC (l) | 4.6 (1.0) | 4.5 (1.0) | 0.284 |
| Atopic (%) | 23.5 | 23.2 | 0.794 |
| FEV1/FVC ⩾0.70 (%) | 92.4 | 90.8 | 0.066 |
| Bronchial hyperresponsiveness (%) | 16.7 | 16.8 | 0.928 |
| Geometric mean methacholine dose‐response slope* | 1.1 | 1.1 | 0.860 |
| Severe respiratory infection as an infant (%) | 7.5 | 6.1 | 0.081 |
| No professional education (%) | 13.3 | 22.0 | <0.001 |
| Exposed to dust and fumes at work (%) | 31.1 | 36.2 | <0.001 |
| Mother smoked (%) | 12.5 | 15.1 | 0.012 |
| Father smoked (%) | 53.9 | 56.5 | 0.085 |
| Current smokers (%) | 31.8 | 40.6 | <0.001 |
| Geometric mean pack‐years in current smokers | 11.2 | 12.8 | 0.071 |
| Never smokers (%) | 45.5 | 36.3 | <0.001 |
| Physician‐diagnosed asthma (%) | 5.5 | 6.9 | 0.050 |
| Wheeze in last 12 months without cold (%) | 6.1 | 8.8 | 0.001 |
| Shortness of breath while walking (%) | 21.8 | 27.7 | <0.001 |
| Chronic cough (%) | 4.1 | 6.0 | 0.002 |
| Chronic phlegm (%) | 5.6 | 8.6 | <0.001 |
*Percentage decrease in FEV1 from its maximum level per µmol methacholine.
Demographic characteristics, respiratory symptoms, and lung function measured in 1991 in participants without BHR compared with hyperreactive symptomatic or asymptomatic persons are shown in table 2. In 1991, 970/5825 (17%) subjects had BHR, of which 492/970 (51%) were asymptomatic. The proportion of women was almost 20% higher than men in both BHR groups, and symptomatic individuals with BHR were more likely to be current smokers. The prevalence of atopy was higher in subjects with BHR, especially when symptomatic. In individuals with BHR, particularly in those with respiratory symptoms, the proportion with abnormal lung function and the degree of functional impairment was slightly higher. As expected, FEV1, FEV1/FVC, and methacholine dose‐response slope showed a trend across categories with the poorest results in individuals with symptomatic BHR.
Table 2 Symptoms and lung function at baseline by presence or absence of bronchial hyperresponsiveness (BHR).
| Baseline measures | No BHR (n = 4855) | BHR | p value* | |
|---|---|---|---|---|
| Silent (n = 492) | Symptomatic (n = 478) | |||
| Proportion of total population (%) | 83 | 9 | 9 | – |
| Sex (% female) | 47 | 67 | 64 | <0.001 |
| Mean (SD) age (years) | 40 (11) | 40 (11) | 41 (12) | 0.41 |
| Current smokers (%) | 31 | 29 | 40 | <0.002 |
| Never smokers (%) | 46 | 51 | 38 | 0.0001 |
| Atopic | 20.8 | 32.3 | 42.3 | <0.001 |
| Physician‐diagnosed asthma (%) | 3.2 | – | 34.3 | – |
| Wheeze in last 12 months without cold (%) | 4.7 | – | 26.8 | – |
| Shortness of breath while walking (%) | 20.3 | – | 60.0 | – |
| Chronic cough (%) | 3.5 | – | 14.0 | – |
| Chronic phlegm (%) | 5.3 | – | 15.1 | – |
| COPD† (%) | 5.8 | 13.4 | 12.8 | <0.001 |
| Mean (SD) FEV1 (% pred)31 | 103 (11) | 96 (11) | 93 (12) | <0.001 |
| Mean (SD) FVC (% pred) | 102 (12) | 100 (12) | 97 (13) | <0.001 |
| Mean (SD) FEV1/FVC | 80 (6) | 78 (7) | 77 (8) | <0.001 |
| FEV1/FVC <0.7 (%) | 6 | 13 | 20 | <0.001 |
| Geometric mean methacholine dose‐response slope‡ | 0.7 | 6.5 | 10.0 | <0.001 <0.001 |
*Significance test between individuals without BHR and individuals with asymptomatic BHR.
†COPD was defined as FEV1/FVC <0.70 and no physician's diagnosis of asthma at either survey.
‡Percentage decline in FEV1 per μmol methacholine relative to maximum FEV1.
Longitudinal results and multivariate analyses
Longitudinal results are given in table 3. Reported new symptoms at SAPALDIA 2 in previously asymptomatic participants with and without BHR are compared. Participants with BHR were at greater risk of developing respiratory symptoms and asthma, as well as COPD.
Table 3 New reports of respiratory symptoms and prevalence of COPD at SAPALDIA 2 in formerly asymptomatic participants with and without BHR at SAPALDIA 1.
| Symptoms developed between surveys | Asymptomatic at baseline | p value | |
|---|---|---|---|
| No BHR (n = 3439) | BHR (n = 492) | ||
| Physician‐diagnosed asthma (%) | 2.0 | 5.7 | <0.001 |
| Wheeze in last 12 months without cold (%) | 3.4 | 8.3 | <0.001 |
| Shortness of breath while walking (%) | 11.9 | 19.1 | <0.001 |
| Chronic cough (%) | 2.3 | 5.9 | 0.002 |
| Chronic phlegm (%) | 4.8 | 4.9 | 0.964 |
| COPD* (%) | 14.3 | 37.9 | <0.001 |
*COPD was defined as FEV1/FVC <0.70 and no physician's diagnosis of asthma.
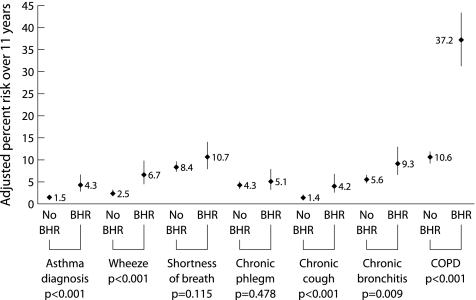
The results of multivariate logistic regression analysis are presented in table 4 and fig 1. Participants diagnosed with asthma between 1991 and 2002 were excluded from analyses for chronic cough, phlegm, and COPD, allowing us to focus on the role of BHR in the onset of those conditions. Silent BHR conferred an increased risk of newly diagnosed asthma, new symptoms of wheeze, chronic cough, and COPD 11 years later. Excluding subjects with COPD in 1991 reduced the association between the presence of BHR and COPD in 2002 slightly, but a significant association remained (adjusted OR 4.0, 95% CI 2.9 to 5.6, p<0.001). There was no relation with new reports of chronic phlegm. Despite the differences observed in the prevalence of BHR between sexes at SAPALDIA 1, there was no evidence of interaction by sex in the effect of BHR on symptoms. However, there is some evidence for sex differences in the association between the presence of BHR and the risk of COPD (p = 0.087), with slightly lower risks in women (OR 4.1 (95% CI 2.8 to 6.1) than in men (OR 5.4 (95% CI 3.4 to 8.5).
Table 4 Risk for the development of respiratory symptoms and for the presence of COPD at SAPALDIA 2 related to bronchial hyperresponsiveness (BHR) in asymptomatic individuals.
| Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | p value† | |
|---|---|---|---|
| Asthma phenotypes | |||
| Physician‐diagnosed asthma | 3.0 (1.9 to 4.7) | 3.0 (1.8 to 5.0) | <0.001 |
| Wheeze in last 12 months without cold | 2.7 (1.8 to 3.9) | 2.9 (1.8 to 4.5) | <0.001 |
| Shortness of breath while walking | 1.8 (1.4 to 2.4) | 1.3 (0.9 to 1.8) | 0.115 |
| COPD phenotypes‡ | |||
| All subjects | |||
| Chronic phlegm | 1.0 (0.7 to 1.6) | 1.2 (0.7 to 2.0) | 0.478 |
| Chronic cough | 2.7 (1.7 to 4.3) | 3.0 (1.7 to 5.2) | <0.001 |
| Chronic bronchitis¶ | 3.0 (1.5 to 6.3) | 2.6 (1.1 to 6.0) | 0.023 |
| COPD§ | 3.7 (2.9 to 4.7) | 4.5 (3.3 to 6.0) | <0.001 |
OR, odds ratio; CI, confidence interval.
*From logistic regression with adjustments for sex, age, FVC in 1991, BMI in 1991, change in weight, exposure to environmental tobacco smoke reported in 2002, smoking status in 2002, pack years in 2002, atopy at baseline, exposure to dust and fumes at work in 2002, level of education at baseline, and study area.
†For effect estimates in adjusted analyses.
‡Participants diagnosed with asthma between 1991 and 2002 were excluded from analyses for chronic cough, phlegm, chronic bronchitis, and COPD.
§COPD was defined as FEV1/FVC <0.70 and no physician's diagnosis of asthma.
¶Chronic bronchitis defined as the presence of chronic cough or phlegm.
Figure 1 Adjusted risk for subsequent respiratory symptoms, asthma and COPD among subjects who were symptom‐free at baseline according to the presence or absence of BHR. Estimates are derived from a logistic regression model upon adjusting covariates listed in the footnote to table 4 to their mean values. Participants diagnosed with asthma between 1991 and 2002 were excluded from the analysis of chronic cough, chronic phlegm, chronic bronchitis, and COPD.
The associations between responsiveness and the clinical phenotypes persisted when responsiveness was quantified by slope. There were highly significant non‐linear relations between methacholine slope and new wheeze, physician‐diagnosed asthma, and COPD (all p<0.01), whereas new symptoms of cough, phlegm, or shortness of breath were not associated with the degree of bronchial responsiveness. There was a non‐linear relation between methacholine slope at SAPALDIA 1 and change in FEV1/FVC over the follow up period in men and a linear relation in women (p<0.001 for differences between men and women).
Silent BHR and decline in FEV1
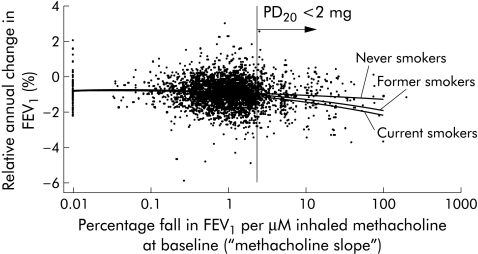
In asymptomatic individuals, BHR was associated with an accelerated decline in FEV1. Our linear regression analyses adjusting for potential confounders (from baseline assessment: FEV1, age, age squared, height; change between surveys: weight; from the follow up assessment: exposure to environmental tobacco smoke and exposure to dust and fumes at work) showed that this effect was significantly modified by smoking status (p = 0.02), but not by sex (p = 0.17). Silent BHR was associated with an additional decline in FEV1 by 12 (5–18) ml/year (p = 0.038), 11 (5–16) ml/year (p<0.001), and 4 (2–8) ml/year (p<0.001) in current smokers, former smokers, and never smokers, respectively, at SAPALDIA 2 compared with asymptomatic participants without BHR. Figure 2 shows the relationship of the observed annual loss of FEV1 as a function of the methacholine response slope. Although the variability in the observed change in FEV1 over time is significant, the modelled adjusted mean follows the same lines—current and former smokers with BHR have a greater loss in FEV1 than lifelong non‐smokers.
Figure 2 Relative annual change in FEV1 (expressed as percentage of baseline) and responsiveness to methacholine in 1991. The scatter plot shows the unadjusted relative annual change in FEV1 in participants who underwent a bronchial challenge at baseline and spirometric tests at both surveys. The line plot shows the mean annual change in FEV1 by methacholine slope adjusted for FEV1 at baseline, sex, height, pack years, exposure to environmental tobacco smoke, area, weight change, and occupation exposure to dust and fumes by smoking status at SAPALDIA 2. The vertical line indicates the PD20 at 2 mg methacholine and thus separates participants with and without bronchial hyperresponsiveness to methacholine.
Discussion
This prospective population based study confirms that BHR is associated with the development of respiratory symptoms, asthma, and COPD. Active smoking in individuals with BHR conferred a synergistic detrimental effect on the loss of lung function. The effects were observed in a population defined as asymptomatic at baseline.
Interpretation of our results warrants careful consideration. Some selection bias cannot be excluded since participation in the bronchial challenge required fulfilment of health criteria as well as satisfactory spirometry. Non‐participants in the follow up survey who had a challenge at baseline were significantly more likely to be smokers, symptomatic, and of lower educational background, although they were similar to participants in terms of lung function characteristics.
The definitions of asthma and COPD are controversial, especially in the context of epidemiological studies.26,32 In order to address the problems of misclassification in reports of new symptoms, we used a rather sensitive definition for asymptomatic (absence of all symptoms) at baseline to exclude as many participants as possible with undetected but existing respiratory symptoms. On the other hand, we used more specific definitions of symptoms at follow up which should have minimised false positive reports of new symptoms.
In addition, we found significant dose‐response relations between new symptoms and BHR as a continuous variable. The coherence of our findings strongly supports the importance of BHR as a risk factor for the development of asthma as well as COPD, and argues against misclassification explaining the results. An additional potential limitation to the study is the fact that we did not measure post‐bronchodilator lung function. As a consequence, our definition of COPD may be somewhat imprecise because we may have underestimated lung volumes in participants with reversible airway obstruction. However, we have tried to address this possible bias by excluding all individuals with physician‐diagnosed asthma.33
BHR at baseline was associated with an increased risk of COPD (defined as FEV1/FVC <0.70) at follow up. This observation confirms the finding of a longitudinal Dutch study8,9 which found a positive association between BHR and the development of respiratory symptoms, and a negative association with the resolution of such symptoms. However, in their study subjects with asthma were not systematically excluded from the primary analyses. In order to reduce the risk of contamination between asthmatic and COPD phenotypes, we excluded subjects with physician‐diagnosed asthma when assessing BHR as a predictor for COPD. In the multivariate analyses we also adjusted for FVC, since responsiveness is affected by lung size and airway calibre.6,29
Since individuals with BHR at baseline had an increased risk for COPD at follow up, BHR may precede the development of COPD and not be just a consequence of it. The highest annual losses of FEV1 were observed in current smokers with BHR, which suggests that BHR is not only an independent risk factor for the development of COPD but also increases the detrimental effect of cigarette smoking.
Interestingly, the same holds true for asthma: BHR is an independent risk factor for the development of asthma. There is good evidence of an interaction between BHR and airway inflammation derived from cross sectional and longitudinal studies as well as from pharmacological intervention trials.34,35,36 The prevalence of atopy was higher in subjects with BHR at baseline, especially when symptomatic. However, in our adult study population aged 30–72 years, there was no evidence of a modification of the effect of BHR by atopy. It could be that atopy plays a more important role in the development of respiratory disease in younger adults and children than later in life.37
The common mechanism triggering the interaction between BHR and either the (atopic) inflammation leading to asthma or the smoke induced inflammation leading to COPD remains speculative. The abnormal airflow resulting from BHR might alter the deposition profile of both allergen and cigarette smoke derived particles in the central and peripheral airways. Indeed, Kohlhäufl et al38 found that women with BHR have increased deposition of fine particles compared with women without BHR, independent of their smoking habits. As a result of an increased exposure to allergen derived particles, sensitisation to airborne allergens would be more likely. Different studies have shown a dose‐response relation between allergen exposure and sensitisation rates.39,40 In addition, once sensitised, the ongoing increased exposure fuels atopic airway inflammation.36 Similarly, increased airway deposition of cigarette smoke derived particles could increase local toxicity and gradually worsen airway inflammation and dysfunction. There is evidence from studies in bronchial biopsies41 and sputum markers42 that, even in individuals with asymptomatic BHR, there are signs of active inflammation. The dose‐response relation between the quantity of cigarettes smoked and BHR,43 airway inflammation,44 and the risk for COPD45 are well known and overtly visible in daily clinical practice. In fact, an altered deposition profile would render subjects with BHR more vulnerable to any sort of particulate inhalation irritants. Further investigations are needed to analyse whether subjects with BHR are more vulnerable to air pollutants in general.
Women had a 20% higher prevalence of BHR at baseline, which is in line with the findings of the ECRHS study.20 There was no evidence in this population sample of a sex difference in the effect of BHR on the development of symptoms 11 years later. However, there were some differences in the effect of BHR on decline in FEV1/FVC between men and women, with slightly stronger effects observed in men.
In conclusion, BHR is a risk factor for the development of respiratory symptoms, asthma and COPD, and is associated with an increased annual loss of FEV1. Particularly at risk for COPD are active smokers with BHR. The combination of BHR and smoking confers a detrimental synergistic effect on the decline in FEV1. Further studies are needed to elucidate the exact pathogenesis underlying this phenomenon.
Acknowledgements
The study could not have been conducted without the help of the study participants, technical and administrative support and the medical teams and field workers at the local centres. We particularly thank the SAPALDIA participants and their continued participation. Local fieldworkers: Aarau: M Broglie, M Bünter, G Drita; Basle: R Armbruster, T Damm, M Gut, L Maier, A Vögelin, L Walter; Davos: D Jud; Geneva: M Ares, M Bennour, B Galobardes, E Namer; Lugano: B Baumberger, S Boccia Soldati, E Gehrig‐Van Essen, S Ronchetto; Montana: C Bonvin; Payerne: S Blanc, A V Ebinger, M L Fragnière, J Jordan; Wald: N Kourkoulos, U Schafroth. Software technicians: S Baur, P Frankenbach, D Burkhard. Administrative assistants: D Baehler, N Bauer, R Nilly.
Abbreviations
BHR - bronchial hyperresponsiveness
COPD - chronic obstructive pulmonary disease
FEV1 - forced expiratory volume in 1 second
FVC - forced vital capacity
Footnotes
The SAPALDIA study was supported by the National Science Foundation of Switzerland (grant no 32‐65896.01, NF 32‐59302.99, 3247B0‐102081), the Federal Office for Forest, Environment and Landscape, the Federal Office of Public Health, the Federal Office of Roads and Transport, the Cantons Basel‐Stadt, Basel‐Land, Geneva, Zurich, Ticino, Aargau, Luzern, the Swiss Lung League and the Lung League of Ticino, Zurich and Basel Stadt/Basel Landschaft. SAPALDIA Basel is part of the European Respiratory Health Survey.
Competing interests: none declared.
References
- 1.Koskela H O, Hyvarinen L, Brannan J D.et al Responsiveness to three bronchial provocation tests in patients with asthma. Chest 20031242171–2177. [DOI] [PubMed] [Google Scholar]
- 2.Magnussen H, Richter K, Taube C. Are chronic obstructive pulmonary disease (COPD) and asthma different diseases? Clin Exp Allergy 199828(Suppl 5)187–94 2035. [DOI] [PubMed] [Google Scholar]
- 3.Sparrow D, O'Connor G, Colton T.et al The relationship of nonspecific bronchial responsiveness to the occurrence of respiratory symptoms and decreased levels of pulmonary function. The Normative Aging Study. Am Rev Respir Dis 19871351255–1260. [DOI] [PubMed] [Google Scholar]
- 4.Woolcock A J, Peat J K, Salome C M.et al Prevalence of bronchial hyperresponsiveness and asthma in a rural adult population. Thorax 198742361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burney P G, Britton J R, Chinn S.et al Descriptive epidemiology of bronchial reactivity in an adult population: results from a community study. Thorax 19874238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz J, Schindler C, Zemp E.et al Predictors of methacholine responsiveness in a general population. Chest 2002122812–820. [DOI] [PubMed] [Google Scholar]
- 7.Jansen D F, Timens W, Kraan J.et al (A)symptomatic bronchial hyper‐responsiveness and asthma. Respir Med 199791121–134. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Rijcken B, Schouten J P.et al Airways responsiveness and development and remission of chronic respiratory symptoms in adults. Lancet 19973501431–1434. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, Prescott E. Update on the “Dutch hypothesis” for chronic respiratory disease. Thorax 199853(Suppl 2)S15–S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holgate S T. The cellular and mediator basis of asthma in relation to natural history. Lancet 1997350(Suppl 2)SII5–SII9. [DOI] [PubMed] [Google Scholar]
- 11.Roth M, Johnson P R, Borger P.et al Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth‐muscle cells. N Engl J Med 2004351560–574. [DOI] [PubMed] [Google Scholar]
- 12.Jogi R, Janson C, Boman G.et al Bronchial hyperresponsiveness in two populations with different prevalences of atopy. Int J Tuberc Lung Dis 200481180–1185. [PubMed] [Google Scholar]
- 13.Abramson M, Kutin J J, Raven J.et al Risk factors for asthma among young adults in Melbourne, Australia. Respirology 19961291–297. [DOI] [PubMed] [Google Scholar]
- 14.Brutsche M, Britschgi D, Dayer E.et al Exercise‐induced bronchospasm (EIB) in relation to seasonal and perennial specific IgE in young adults. Allergy 199550905–909. [DOI] [PubMed] [Google Scholar]
- 15.Segala C, Korobaeff M, Maccario J.et al Bronchial hyperresponsiveness as a predictor of wheezing in a follow‐up study of healthy men. Respiration 199663352–357. [DOI] [PubMed] [Google Scholar]
- 16. Bateman , ed. Using clinical measures of disease control to reduce the burden of asthma. Pharmacoeconomics 200119(Suppl 2)7–12. [DOI] [PubMed] [Google Scholar]
- 17.Jeffery P K, Godfrey R W, Adelroth E.et al Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am Rev Respir Dis 1992145890–899. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor G T, Sparrow D, Weiss S T. Normal range of methacholine responsiveness in relation to prechallenge pulmonary function. The Normative Aging Study. Chest 1994105661–666. [DOI] [PubMed] [Google Scholar]
- 19.Wise R A, Kanner R E, Lindgren P.et al The effect of smoking intervention and an inhaled bronchodilator on airways reactivity in COPD: the Lung Health Study. Chest 2003124449–458. [DOI] [PubMed] [Google Scholar]
- 20.Chinn S, Jarvis D, Luczynska C M.et al An increase in bronchial responsiveness is associated with continuing or restarting smoking. Am J Respir Crit Care Med 2005172156–161. [DOI] [PubMed] [Google Scholar]
- 21.Sparrow D, O'Connor G T, Rosner B.et al Predictors of longitudinal change in methacholine airway responsiveness among middle‐aged and older men: the Normative Aging Study. Am J Respir Crit Care Med 1994149376–381. [DOI] [PubMed] [Google Scholar]
- 22.Willemse B W, ten Hacken N H, Rutgers B.et al Association of current smoking with airway inflammation in chronic obstructive pulmonary disease and asymptomatic smokers. Respir Res 2005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin B W, Ackermann‐Liebrich U, Leuenberger P.et al SAPALDIA: methods and participation in the cross‐sectional part of the Swiss Study on Air Pollution and Lung Diseases in Adults. Soz Praventivmed 19974267–84. [DOI] [PubMed] [Google Scholar]
- 24.Ackermann‐Liebrich U, Kuna‐Dibbert B, Probst‐Hensch N M.et al Follow‐up of the Swiss Cohort study on Air Pollution and Lung Diseases in Adults (SAPALDIA 2) 1991–2003: Methods and characterisation of participants. Soz Praventivmed 200550245–263. [DOI] [PubMed] [Google Scholar]
- 25.Burney P G, Luczynska C, Chinn S.et al The European Community Respiratory Health Survey. Eur Respir J 19947954–960. [DOI] [PubMed] [Google Scholar]
- 26.Pauwels R A, Buist A S, Calverley P M.et al Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 20011631256–1276. [DOI] [PubMed] [Google Scholar]
- 27.Kuna‐Dibbert B, Künzli N, Keidel D.et al Longitudinal validity of spirometers ‐ a challenge in lung function follow‐up studies. Swiss Med Wkly 2005135503–508. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor G, Sparrow D, Taylor D.et al Analysis of dose‐response curves to methacholine. An approach suitable for population studies. Am Rev Respir Dis 19871361412–1417. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti P, Carrozzi L, Viegi G.et al Distribution of bronchial responsiveness in a general population: effect of sex, age, smoking, and level of pulmonary function. Am J Respir Crit Care Med 19951511770–1777. [DOI] [PubMed] [Google Scholar]
- 30.Quanjer P H, Tammeling G J, Cotes J E.et al Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993165–40. [PubMed] [Google Scholar]
- 31.Brandli O, Schindler C, Kunzli N.et al Lung function in healthy never smoking adults: reference values and lower limits of normal of a Swiss population. Thorax 199651277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pekkanen J, Pearce N. Defining asthma in epidemiological studies. Eur Respir J 199914951–957. [DOI] [PubMed] [Google Scholar]
- 33.Vestbo J. COPD in the ECRHS. Thorax 20045989–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabe K F. Mechanisms of immune sensitization of human bronchus. Am J Respir Crit Care Med 1998158S161–S170. [DOI] [PubMed] [Google Scholar]
- 35.Bootsma G P, Dekhuijzen P N, Festen J.et al Comparison of fluticasone propionate and beclomethasone dipropionate on direct and indirect measurements of bronchial hyperresponsiveness in patients with stable asthma. Thorax 1995501044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henriksen A H, Sue‐Chu M, Holmen T L.et al Exhaled and nasal NO levels in allergic rhinitis: relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur Respir J 199913301–306. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell A R, Toelle B G, Marks G B.et al Age‐specific relationship between CD14 and atopy in a cohort assessed from age 8 to 25 years. Am J Respir Crit Care Med 2004169615–622. [DOI] [PubMed] [Google Scholar]
- 38.Kohlhaufl M, Brand P, Scheuch G.et al Increased fine particle deposition in women with asymptomatic nonspecific airway hyperresponsiveness. Am J Respir Crit Care Med 1999159902–906. [DOI] [PubMed] [Google Scholar]
- 39.Sporik R, Holgate S T, Platts‐Mills T A.et al Exposure to house‐dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med 1990323502–507. [DOI] [PubMed] [Google Scholar]
- 40.Lau S, Illi S, Platts‐Mills T A.et al Longitudinal study on the relationship between cat allergen and endotoxin exposure, sensitization, cat‐specific IgG and development of asthma in childhood: report of the German Multicentre Allergy Study (MAS 90). Allergy 200560766–773. [DOI] [PubMed] [Google Scholar]
- 41.Laprise C, Laviolette M, Boutet M.et al Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodelling. Eur Respir J 19991463–73. [DOI] [PubMed] [Google Scholar]
- 42.Betz R, Kohlhaufl M, Kassner G.et al Increased sputum IL‐8 and IL‐5 in asymptomatic nonspecific airway hyperresponsiveness. Lung 2001179119–133. [DOI] [PubMed] [Google Scholar]
- 43.Rijcken B, Schouten J P, Mensinga T T.et al Factors associated with bronchial responsiveness to histamine in a population sample of adults. Am Rev Respir Dis 19931471447–1453. [DOI] [PubMed] [Google Scholar]
- 44.Hogg J C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004364709–721. [DOI] [PubMed] [Google Scholar]
- 45.Lindberg A, Jonsson A C, Ronmark E.et al Ten‐year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest 20051271544–1552. [DOI] [PubMed] [Google Scholar]