Abstract
Two of the genes defective in the five complementation groups identified in the class II-negative bare lymphocyte syndrome or corresponding laboratory mutants have been cloned. One gene encodes a protein, RFX5, that is a member of the RFX family of DNA binding proteins. The other, CIITA, encodes a large protein with a defined acidic transcriptional activation domain; this protein does not interact with DNA. Expression plasmids encoding regions of RFX5 fused to the GAL4 DNA binding domain activated transcription from a reporter construct containing GAL4 sites in a cotransfection assay in the Raji human B cell line. However, these plasmids produced transcriptional activity in HeLa cells only in conjunction with interferon γ stimulation, a condition in which expression of both CIITA and class II major histocompatibility complex surface proteins are induced. Furthermore, these plasmids were not active in RJ2.2.5, an in vitro mutagenized derivative of Raji in which both copies of CIITA are defective. Transcriptional activation by the RFX5 fusion protein could be restored in RJ2.2.5 by cotransfection with a CIITA expression plasmid. Finally, a direct interaction between RFX5 and CIITA was detected with the yeast two-hybrid and far-Western blot assays. Thus, RFX5 can activate transcription only in cooperation with CIITA. RFX5 and CIITA associate to form a complex capable of activating transcription from class II major histocompatibility complex promoters. In this complex, promoter specificity is determined by the DNA binding domain of RFX5 and the general transcription apparatus is recruited by the acidic activation domain of CIITA.
Type II combined immunodeficiency or bare lymphocyte syndrome (BLS) is a rare congenital disease characterized by a lack of transcription of major histocompatibility complex (MHC) class II genes (1). BLS patients fall into three genetic complementation groups and an in vitro mutant defined a fourth (2–5). Recently, a potential fifth complementation group has been reported. The atypical phenotype of these latter patients exhibited discoordinate regulation between α and β chains and low levels of class II surface expression on some cell types (6, 7). Transacting factors are responsible for these various defects since fusion of B cell lines derived from patients with those from a patient representing a different complementation group or with a normal cell line restores class II expression. The defective genes in two of the complementation groups have been cloned. Class II MHC transactivator (CIITA) is defective in patients from complementation group A (8). Transfection experiments with plasmids expressing wild-type CIITA rescued MHC class II expression in three cell lines from this group (8). Furthermore, cell lines from this group carry mutations in both copies of the CIITA gene. Unlike other factors reported to regulate class II MHC transcription for which genes have been cloned and which have all been shown to be ubiquitously transcribed DNA binding proteins (9, 10), the message for CIITA correlates with class II MHC expression in cell lines (8). Furthermore, this correlation extends to interferon γ (IFN-γ)-inducible expression and developmental extinction in plasma cells since in both cases induced expression of CIITA alone causes class II MHC expression (11, 12). Thus, these results strongly suggest that while several transcription factors are required for class II MHC expression, CIITA is the specific regulator that controls both developmental and inducible class II MHC gene expression.
CIITA does not bind DNA but does contain two functional domains. The N terminus encodes an acidic transcriptional activator, and the C terminus targets the protein to MHC class II promoters (13). The acidic activator is not specific for class II transcription and the function of this domain can be replaced with an activation domain from a viral protein (13, 14).
The gene for RFX5, defective in complementation group B, was cloned by the same approach as CIITA (15). RFX5 shares homology with a family of DNA binding proteins (RFX1–4), a member of which was initially misidentified as the factor responsible for the BLS defect (16) but was subsequently reported to regulate hepatitis B virus enhancer activity (17, 18). Two B cell lines derived from BLS patients in complementation group B have defective RFX5 loci on both chromosomes, and class II MHC expression can be rescued in these lines by transfection of an expression plasmid encoding wild-type RFX5 (15). The role of RFX5 in class II transcription is also supported by its specific binding to a conserved class II promoter element, the X box, and its presence in nuclear complexes which form on the DRA promoter in electrophoretic mobility shift assay (15).
Herein, deletion mutagenesis demonstrates the regions of RFX5 that are required for activating transcription in a B cell line. However, in HeLa cells this activity is detected only after IFN-γ stimulation. Furthermore, in a cell that is defective in CIITA, this region of RFX5 is only functional after genetic complementation with a CIITA expression plasmid. Finally, a direct interaction between RFX5 and CIITA was detected in vivo with the yeast two-hybrid assay and in vitro by direct protein–protein interaction with a far-Western blot analysis. Thus, RFX5 activates transcription indirectly by recruitment of CIITA specifically to the class II MHC promoter where the previously characterized N-terminal acidic domain of CIITA serves to activate transcription.
MATERIALS AND METHODS
Isolation of CIITA and RFX5 cDNAs and Construction of Plasmids.
Raji cDNA was prepared by commercial mRNA isolation (Oligotex, Qiagen, Chatsworth, CA) and synthesis kits (Stratascript, Stratagene). This cDNA and oligonucleotides (GIBCO/BRL) for CIITA, CIITA.1 (ATAGAATTCGCAGCTGGAGAGGTCTCCAAC; contains EcoRI adapter underlined) and CIITA.2 (ATACTCGAGTCTCAGGCTGATCCGTGAATC; contains XhoI adapter underlined); for RFX5, RFX5.1 (GTGGCTACGAGTTTTCCAGATT) and RFX5.2 (GGAATACAGGTAAGGTCACTAC) were employed in the PCR using pfu DNA polymerase (Stratagene) to isolate the CIITA and RFX5 coding regions. These fragments were cloned into the EcoRI and XhoI sites of pBluescript II KS+ for CIITA and designated pBSCIITA or cloned into the SmaI site for RFX5 and designated pBSRFX5. For mammalian expression and the yeast two-hybrid system, the CIITA coding region was inserted into pBKCMV (Stratagene) from which the lac promoter had been deleted and designated pCMVCIITA and into pGAD424 (CLONTECH) in frame with the Gal4 activation domain and designated pGADCIITA.
Deletions of the RFX5 coding sequence were cloned in frame with the GAL4 DNA binding domain of pBXG1 using restriction sites KpnI (bp 273) for essentially full-length pGALRFXΔ34, XhoI (bp 750) and PstI (bp 1125) without the DNA binding domain for pGALRFXΔ194 and pGALRFXΔ318, and EcoRI (bp 1600) without the proline-rich region for pGALRFXΔ477. The RFX5 deletions resulting from digestion with KpnI, XhoI, and PstI were also inserted into the yeast two-hybrid expression vector pGBT9 and designated pGBRFXΔ34, pGBRFXΔ194, and pGBRFXΔ318. For in vitro translation the XhoI and PstI derivatives of RFX5 were inserted into pCITE2a (Novagen) and designated pTLNRFX5Δ194 and pTLNRFX5Δ318. For bacterial expression, CIITA regions corresponding to residues 1–161 and 299–1129 were cloned into pET15b (Novagen) and designated pHISCIITTA1–161 and pHISCIITA299–1129. The plasmid pFUSE contains the GAL4 DNA binding domain (residues 1–147) in pET15b.
All PCR products were completely sequenced, as were all junctions in cloning operations (Sequenase, United States Biochemical). Plasmids pG5EC, pBXG1, pBXGAL-VP, and pFUSE were the generous gift of Mark Ptashne. Plasmids pTD-1 and pVa3 were purchased (CLONTECH).
Cell Culture and Transfection.
Raji (CCL 86, American Type Culture Collection) is a Burkitt lymphoma B cell line that expresses all MHC class II isotypes. RJ2.2.5 was derived by γ-irradiation mutagenesis of Raji and is defective for global MHC class II transcription (19). The HeLa cell line (CCL 2, American Type Culture Collection) was derived from a cervical carcinoma. Cells were cultured and treated with IFN-γ as reported (20).
Cells were transfected with the indicated amounts of plasmids by electroporation. Briefly, cells were washed twice in PBS with 10 mM Hepes (pH 7.3) and resuspended at 4 × 107 cells per ml, on ice. Aliquots of 1 × 107 cells were mixed with DNA, pulse-labeled at 250 V and 960 μFd, maintained on ice for 10 min, and plated in 100-mm dishes with 15 ml of medium. After 48 hr of culture, chloramphenicol acetyltransferase activities were determined as reported (21). Quantitative measurements of different chloramphenicol species using duplicate independent transfections of each sample were made with phosphoimaging equipment (Fuji). As an internal control of transfection efficiencies 2 μg of pCMVβ (22) was included in all transfections. β-Galactosidase activity was measured spectrophotometrically. This result showed that transfection efficiency generally varied less than 10% and always less than 15%; therefore, no normalization to this control was done.
Southern Blot Analysis.
CIITA mRNA was assessed in Southern blots of PCR products from cDNA (prepared as above) using primers CIITA.2 and CIITA.3 (TTGAGCGACACGGTGGCGCTGTGGGAG). Blots were prepared, hybridized, and washed under high-stringency conditions as described (23).
Yeast Two-Hybrid System and β-Galactosidase Assay.
All yeast manipulations were performed in strain SFY526 in accordance with the suppliers protocols (CLONTECH). All quantitative data was derived from three independent colonies transformed with the indicated plasmid(s). The β-galactosidase assay was performed as described (24).
Far-Western Blot Analysis.
Recombinant proteins from plasmids pHISCIITTA1–161, pHISCIITA299–1129, and pFUSE were induced in host Bl21(DE3) (Novagen) and purified by nickel-chelate chromatography under denaturing conditions for CIITA-(299–1129) and native conditions for CIITA-(1–161) and GAL4-(1–147) (Qiagen, Chatsworth, CA; protocols 7 and 5, respectively). Western blots were made and probed as described (24) except that 5 μg of recombinant protein was loaded per lane and protein probes were 35S-radiolabeled by in vitro translation (TnT reticulocyte, Promega).
RESULTS
RFX5 Activates Transcription When Tethered to a Heterologous Promoter in Raji B Cells.
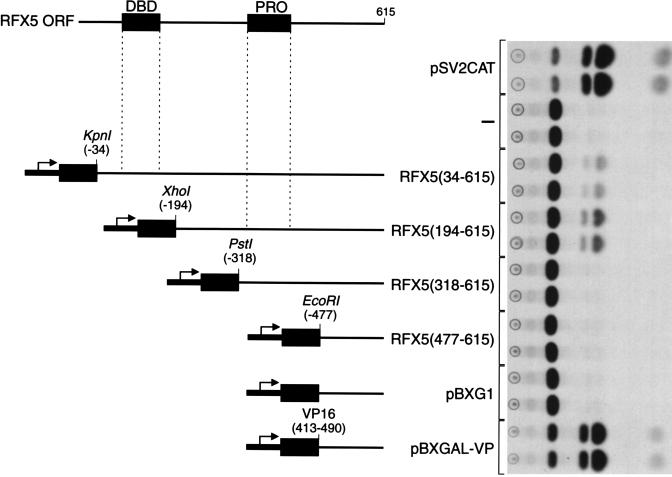
A series of expression plasmids were constructed that fused coding regions of RFX5 to the GAL4 DNA binding domain. The plasmids were designed to remove from the 5′ end successive putative functional domains identified within the RFX5 primary structure including the DNA binding and proline-rich domains and the central region of the coding sequence. These plasmids and the controls, pBXG1, which expresses the GAL4 DNA binding domain, and pBXGAL-VP, which expresses the DNA binding domain fused to the VP16 acidic activator, were cotransfected into Raji B cells with the reporter plasmid pG5EC, which contains the GAL4 binding sites upstream of the chloramphenicol acetyltransferase gene (25). Transcription was activated by the expression of two of these RFX5 fusion proteins corresponding to 5′ deletions at amino acids residues 34 and 194 in the open reading frame (Fig. 1). These results demonstrate that portions of the RFX5 protein can activate transcription within B cells in a context other than that of the class II MHC promoter and that RFX5 can activate transcription from the reporter construct in the absence of other MHC class II promoter binding proteins. Since activity was maintained after deletion of the first 194 amino acids of RFX5, its DNA binding region was not required for transcriptional activation and could be substituted by the GAL4 DNA binding domain. Furthermore, comparison of the activities induced by the 34- and 194-residue deletion constructs indicates that stronger transcription activation was seen with the 194-residue deletion. The presence of the RFX5 DNA binding domain may sequester some of the fusion protein to recognition sites within the genome and away from the reporter plasmid.
Figure 1.
Regions of RFX5 that activate transcription in the Raji human B cell line. A series of 5′ deletions of the RFX5 coding region were fused to the GAL4-(1–147) DNA binding domain. These plasmids were designed to sequentially remove putative functional domains detected in the primary structure of RFX5. RFX5-(34–615) is essentially full-length, RFX5-(194–615) removes the DNA-binding domain, RFX5-(318–615) removes the central region, and RFX5-(477–615) removes the proline-rich region. Numbers used are the numbers of amino acid residues deleted from the reported translation initiation codon. pBXGAL-VP expresses the GAL4 DNA binding domain fused to the acidic activation domain of VP16-(413–490). Cells were transfected with 10 μg of pG5EC alone as a control for background activity by this reporter construct. The autoradiograph presents data from independent duplicate transfections of acetylated chloramphenicol species separated by TLC used to measure reporter gene activity.
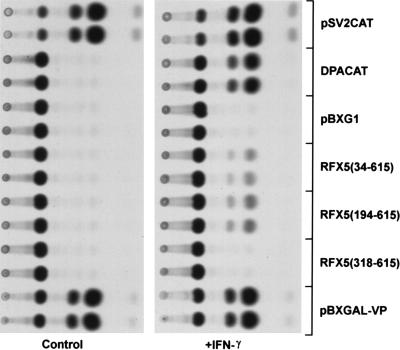
Transcriptional Activation by the RFX5 Activation Domain Is IFN-γ-Inducible in HeLa Cells.
HeLa cells are well characterized as a model system for IFN-γ induction of class II MHC genes (26). When the RFX5 deletion plasmids were transfected into HeLa cells, no transcription from the reporter gene plasmid was detected. However, after treatment with IFN-γ, transcriptional activity in HeLa cells was observed in transfections with both the 34- and 194-residue deletion constructs (Fig. 2). Also, consistent with the data obtained from the Raji cell transfections RFX5-(194–615) produced more reporter gene activity than did RFX5-(34–615). These data suggest that RFX5 does not contain an “activation domain” since previously defined activation domains are constituitively functional. Thus, either the RFX5 domain is modified in the cell after IFN-γ treatment or, alternatively, an additional factor, induced or activated by IFN-γ stimulation, might be required for transcriptinal activation by the RFX5 fusion protein. Relevantly, IFN-γ is known to induce the transcription of CIITA, a factor that is essential for transcription from class II MHC promoters (11). In fact, induction of CIITA message could be detected under the IFN-γ stimulation conditions used in these assays (data not shown; see ref. 11).
Figure 2.
Transcriptional activity by RFX5–GAL4 fusion proteins is induced by IFN-γ in HeLa cells. Autoradiographs of reporter gene activity from transfected control HeLa cells and HeLa cells treated with IFN-γ. Cells were transfected with 10 μg of the indicated plasmids. Samples transfected with GAL4 fusion proteins were cotransfected with 10 μg of pG5EC.
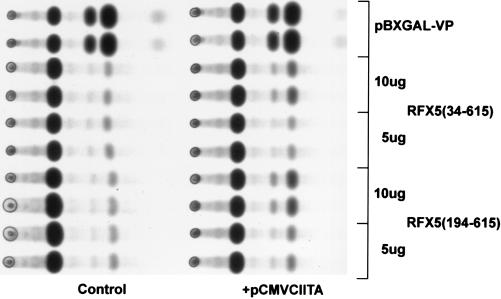
CIITA Is Required for Transcriptional Activation by RFX5.
The cell line RJ2.2.5 was derived by laboratory mutagenesis of the B cell lymphoma line Raji. This line is globally defective in MHC class II transcription due to mutations in both copies of CIITA and serves as a CIITA negative background. Transcriptional activation by RFX5-(34–615) and RFX5-(194–615) was detected in transfection of RJ2.2.5 only when an expression plasmid encoding CIITA was included and not in controls in the absence of CIITA (Fig. 3). Thus, CIITA is required for the activity directed by RFX5.
Figure 3.
Transcriptional activity by RFX4–GAL4 fusion proteins in RJ2.2.5 cells required CIITA. Autoradiographs of reporter gene activity from RJ2.2.5 cells cotransfected with the indicated plasmids and 10 μg of pG5EC and cotransfections that also included 10 μg of pCMVCIITA.
Direct Protein–Protein Interaction Between CIITA and RFX5.
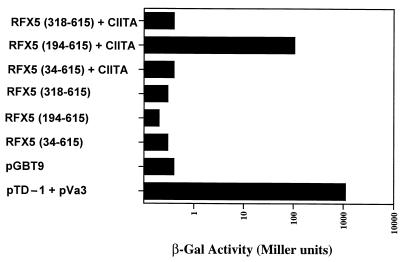
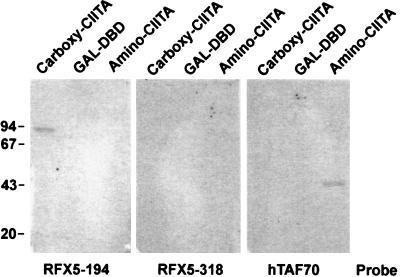
The requirement for CIITA in transcriptional activation mediated through RFX5 could be explained by a direct interaction between these two factors. Two systems were employed to demonstrate this interaction. (i) The yeast two-hybrid system was employed (27). Three of the GAL4 RFX5 inserts in pBXG1 [RFX5-(34–615), -(194–615), and -(318–615)] were cloned into the yeast expression plasmid pGBT9, and the full-length CIITA coding region was inserted into pGAD424. After transformation into yeast, an interaction was detected between CIITA and the fusion protein encoding residues 194–615 of RFX5 (Fig. 4). Unlike the mammalian cotransfection experiments, no activity was detected for the largest RFX5 region corresponding to amino acids 34–615. Furthermore, when yeast were transformed with the pGBT9 derivatives alone, no β-galactosidase activity was detected, confirming that RFX5 cannot activate transcription alone. (ii) Interaction between CIITA and RFX5 was also detected in vitro by the Far-Western technique. Three recombinant proteins that included the N terminus of CIITA (residues 16–147), the C terminus of CIITA (residues 299-1129), and the GAL4 DNA binding domain (residues 1–147) were expressed in bacteria in a vector that encoded an N-terminal histidine affinity tag. The proteins were then purified by nickel-chelate chromatography, refolded on replicate membranes, and probed with 35S-labeled in vitro-translated RFX5 regions RFX5-(194–615) and RFX5-(318–615). As a positive control human TAF70 was included as a probe since it interacts with the N-terminal acidic activation domain of CIITA (28). An interaction in the test samples was detected between the C-terminal region of CIITA, residues 299-1129, and RFX5, residues 194–615, as well as between the N-terminal region of CIITA, residues 1–161, and TAF70 (Fig. 5).
Figure 4.
Interaction between RFX5 and CIITA detected in yeast. Histograms depict β-galactosidase reporter gene activity in yeast extracts from cells transformed with RFX5–GAL4 fusion protein expression plasmids or cotransformation that included a plasmid for expression of CIITA fused to the GAL4 activation domain. All data represent the mean activity detected in triplicate transformants. Individual measurements were within 5% of each other. pGBT9 encodes the GAL4 DNA binding domain alone. pTD-1 used in the positive control encodes a fusion protein made up of amino acids 84–708 of simian virus 40 large tumor antigen and GAL4 activation domain and pVa3 encodes a fusion protein made up of GAL4 DNA binding domain and amino acids 72–350 of p53 with which the simian virus 40T antigen interacts.
Figure 5.
Detection of an interaction between RFX5 and CIITA by far-Western blot analysis. Recombinant proteins (5 μg) were electrophoresed and blotted on quadruplicate gels. Proteins were refolded on the membranes and probed with the indicated radiolabeled in vitro-translated proteins.
DISCUSSION
The isolation of genes encoding transcription factors responsible for the regulation of MHC class II genes by expression and complementation screening techniques permits experiments designed to elucidate the mechanisms through which these proteins function. Initial results from a functional assay using GAL4 fusion proteins suggested that a transcriptional “activation domain” was present in RFX5. However, subsequent data from this system when transfected into other cell types indicated first that this region of RFX5 did not function in all cell types and then that cooperation/interaction with CIITA was required for this activity. Finally, a direct interaction was detected between RFX5 and CIITA both in vivo and in vitro with the yeast two-hybrid assay and biochemically by far-Western blot analysis.
Thus, these results indicate that RFX5 and CIITA can form a complex that is capable of activating transcription. CIITA may function as a coactivator, binding together through protein–protein interactions RFX5 and other class II MHC promoter bound DNA binding proteins including at least those bound to the TATA box. Other interactions may influence the association of CIITA with class II MHC promoters. First, there are multiple cis elements that when mutated reduce transcription from class II MHC promoters, including the X and Y box, the J and S elements, and an octamer element (29, 30). Unless these recognition sites bind factors whose expression specifically correlates with class II transcription, it is likely that they also ultimately function through CIITA. Additionally, cooperative binding of multiple factors to class II promoters has been demonstrated. In fact, CIITA may tie together factors that bind to several DNA elements. In the case of the DRA promoter, the most strongly transcribed of the class II promoters, moreover, an additional proximal element, OCT, recruits the protein OCT2 and, through OCT2, BOB1, which interacts with the TATA box binding protein complex. Synergy in binding may stabilize interactions with RFX5, CIITA, or both (31, 32). In these experiments, the levels of transcription generated by the interaction of the RFX5–GAL4 fusion proteins was much less than that initiated from the DPA proximal promoter (Fig. 2) or that detected when the CIITA activation domain was fused directly to the GAL4 DNA binding region. This finding may be a further indication that additional interaction(s) to those with RFX5 are required for full recruitment of CIITA to or subsequent activation of the MHC promoter.
CIITA is present in higher-order multiprotein complexes present on MHC class II promoters (13), and although it is required for MHC class II transcription and encodes a strong acidic activation domain, it does not bind DNA. Conversely, RFX5 encodes a DNA binding domain that recognizes a DNA element unique to MHC class II genes, the X box. Thus, CIITA functions as a coactivator through specific protein interactions with RFX5. The role for RFX5 in this model is to lend promoter specificity to this complex through X-box-specific recognition by its DNA binding domain. The role of CIITA then is to activate transcription via its N-terminal acidic domain.
Acknowledgments
This work was supported by National Institutes of Health Grant CA47554 and by fellowships of the American Cancer Society to T.S. and of the Irvington Foundation for Medical Research to S.K.M.
ABBREVIATIONS
- IFN-γ
interferon γ
- MHC
major histocompatibility complex
- BLS
bare lymphocyte syndrome
References
- 1.Mach B, Steimle V, Reith W. Immunol Rev. 1994;138:207–221. doi: 10.1111/j.1600-065x.1994.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 2.Benichou B, Strominger J L. Proc Natl Acad Sci. 1991;88:4285–4288. doi: 10.1073/pnas.88.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hume C R, Lee J S. Hum Immunol. 1989;26:288–309. doi: 10.1016/0198-8859(89)90007-4. [DOI] [PubMed] [Google Scholar]
- 4.Hume C R, Shookster L A, Collins N, O’Reilly R, Lee J S. Hum Immunol. 1989;25:1–11. doi: 10.1016/0198-8859(89)90065-7. [DOI] [PubMed] [Google Scholar]
- 5.Yang Z, Acolla R S, Pious D, Zegers B J M, Strominger J L. EMBO J. 1988;7:1965–1972. doi: 10.1002/j.1460-2075.1988.tb03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauber I, Gulle H, Wolf H M, Maris M, Eggenbauer H, Eibl M M. J Exp Med. 1995;181:1411–1423. doi: 10.1084/jem.181.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douhan J, Hauber I, Eibl M M, Glimcher L H. J Exp Med. 1996;183:1063–1069. doi: 10.1084/jem.183.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steimle V, Otten L A, Zufferey M, Mach B. Cell. 1993;75:135–146. [PubMed] [Google Scholar]
- 9.Liou H-C, Boothby M R, Finn P W, Davidon R, Nabavi N, Zeleznik-Le N J, Ting J P-Y, Glimcher L H. Science. 1990;247:1581–1584. doi: 10.1126/science.2321018. [DOI] [PubMed] [Google Scholar]
- 10.Sugawara, M., Scholl, T., Ponath, P. D. & Strominger, J. L. (1994) Mol. Cell. Biol. 14:8438–8450. [DOI] [PMC free article] [PubMed]
- 11.Steimle V, Siegrist C A, Mottet O, Lisowska-Grospierre B, Mach B. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 12.Silacci P, Mottet A, Steimle V, Reith W, Mach B. J Exp Med. 1994;180:1329–1336. doi: 10.1084/jem.180.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley J L, Westerhide S D, Price J A, Brown J A, Boss J M. Immunity. 1995;2:533–543. doi: 10.1016/1074-7613(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H, Glimcher L H. Immunity. 1995;2:545–553. doi: 10.1016/1074-7613(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 15.Steimle V, Durand B, Barras E, Zufferey M, Hadam M R, Mach B, Reith W. Genes Dev. 1995;9:1021–1032. doi: 10.1101/gad.9.9.1021. [DOI] [PubMed] [Google Scholar]
- 16.Reith W, Barras E, Satola S, Kobr M, Reinhart C, Herrero Sanchez C, Mach B. Proc Natl Acad Sci USA. 1989;86:4200–4204. doi: 10.1073/pnas.86.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reith W, Ucla C, Barras E, Gaud A, Durand B, Herrero-Sanchez C, Kobr M, Mach B. Mol Cell Biol. 1994;14:1230–1244. doi: 10.1128/mcb.14.2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegrist C A, Durand B, Emery P, David E, Hearing P, Mach B, Reith W. Mol Cell Biol. 1993;13:6375–6384. doi: 10.1128/mcb.13.10.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Accolla R S. J Exp Med. 1983;157:1053–1058. doi: 10.1084/jem.157.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholl T, Stevens M B, Mahanta S, Strominger J L. J Immunol. 1996;156:1448–1457. [PubMed] [Google Scholar]
- 21.Gorman C M, Moffat L F, Howard B H. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGregor G R, Caskey C T. Nucleic Acids Res. 1989;17:2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholl T, Pitcock A, Jones B. Immunogenetics. 1992;36:255–263. doi: 10.1007/BF00215056. [DOI] [PubMed] [Google Scholar]
- 24.Wu J Y, Maniatis T. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- 25.Sadowski I, Ptashne M. Nucleic Acids Res. 1989;17:7539–7540. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boss J M, Strominger J L. Proc Natl Acad Sci USA. 1986;83:9139–9143. doi: 10.1073/pnas.83.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields S, Song O. Nature (London) 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 28.Mahanta Sanjeev, K, Scholl T, Yang F-C, Strominger J L. Proc Natl Acad Sci USA. 1997;94:6324–6329. doi: 10.1073/pnas.94.12.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glimcher L H, Cara C J. Annu Rev Immunol. 1992;10:13–50. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara M, Scholl T, Ponath P, Strominger J L. Mol Cell Biol. 1994;14:8438–8450. doi: 10.1128/mcb.14.12.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durand B, Kobr M, Reith W, Mach B. Mol Cell Biol. 1994;14:6839–6847. doi: 10.1128/mcb.14.10.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreno C S, Emery P, West J E, Durand B, Reith W, Mach B, Boss J. J Immunol. 1995;155:4313–4321. [PubMed] [Google Scholar]