Abstract
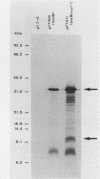
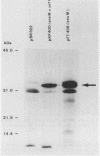
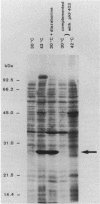
Conjugation and bacteriophage P1 transduction experiments in Escherichia coli showed that resistance to the antibacterial compound diazaborine is caused by an allelic form of the envM gene. The envM gene from Salmonella typhimurium was cloned and sequenced. It codes for a 27,765-dalton protein. The plasmids carrying this DNA complemented a conditionally lethal envM mutant of E. coli. Recombinant plasmids containing gene envM from a diazaborine-resistant S. typhimurium strain conferred the drug resistance phenotype to susceptible E. coli cells. A guanine-to-adenine exchange in the envM gene changing a Gly codon to a Ser codon was shown to be responsible for the resistance character. Upstream of envM a small gene coding for a 10,445-dalton protein was identified. Incubating a temperature-sensitive E. coli envM mutant at the nonpermissive temperature caused effects on the cells similar to those caused by treatment with diazaborine, i.e., inhibition of fatty acid, phospholipid, and lipopolysaccharide biosynthesis, induction of a 28,000-dalton inner membrane protein, and change in the ratio of the porins OmpC and OmpF.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Vagelos P. R. Acetyl CoA carboxylase. I. Requirement for two protein fractions. Proc Natl Acad Sci U S A. 1968 Feb;59(2):561–568. doi: 10.1073/pnas.59.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan A. F., Russell R. R. Conditional mutations affecting the cell envelope of Escherichia coli K-12. Genet Res. 1973 Apr;21(2):139–152. doi: 10.1017/s001667230001332x. [DOI] [PubMed] [Google Scholar]
- Eichenlaub R. Mutants of the mini-F plasmid pML31 thermosensitive in replication. J Bacteriol. 1979 May;138(2):559–566. doi: 10.1128/jb.138.2.559-566.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassberger M. A., Turnowsky F., Hildebrandt J. Preparation and antibacterial activities of new 1,2,3-diazaborine derivatives and analogues. J Med Chem. 1984 Aug;27(8):947–953. doi: 10.1021/jm00374a003. [DOI] [PubMed] [Google Scholar]
- Harder M. E., Ladenson R. C., Schimmel S. D., Silbert D. F. Mutants of Escherichia coli with temperature-sensitive malonyl coenzyme A-acyl carrier protein transacylase. J Biol Chem. 1974 Dec 10;249(23):7468–7475. [PubMed] [Google Scholar]
- Heusser D. Dünnschichtchromatographie von Fettsäuren auf silanisiertem Kieselgel. J Chromatogr. 1968 Mar 4;33(1):62–69. doi: 10.1016/s0021-9673(00)98620-5. [DOI] [PubMed] [Google Scholar]
- Högenauer G., Woisetschläger M. A diazaborine derivative inhibits lipopolysaccharide biosynthesis. Nature. 1981 Oct 22;293(5834):662–664. doi: 10.1038/293662a0. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Godson G. N. Evidence for use of rare codons in the dnaG gene and other regulatory genes of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Feb;80(3):687–691. doi: 10.1073/pnas.80.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. R factor proteins synthesized in Escherichia coli minicells: incorporation studies with different R factors and detection of deoxyribonucleic acid-binding proteins. J Bacteriol. 1974 Dec;120(3):1451–1463. doi: 10.1128/jb.120.3.1451-1463.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D., Staden R., Boswell D. R. A method for measuring the non-random bias of a codon usage table. Nucleic Acids Res. 1984 Dec 21;12(24):9567–9575. doi: 10.1093/nar/12.24.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperka-Gottlieb C. D., Hermetter A., Paltauf F., Daum G. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1988 Dec 22;946(2):227–234. doi: 10.1016/0005-2736(88)90397-5. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wehlmann H., Eichenlaub R. Plasmid mini-F encoded proteins. Mol Gen Genet. 1980;180(1):205–211. doi: 10.1007/BF00267371. [DOI] [PubMed] [Google Scholar]