Abstract
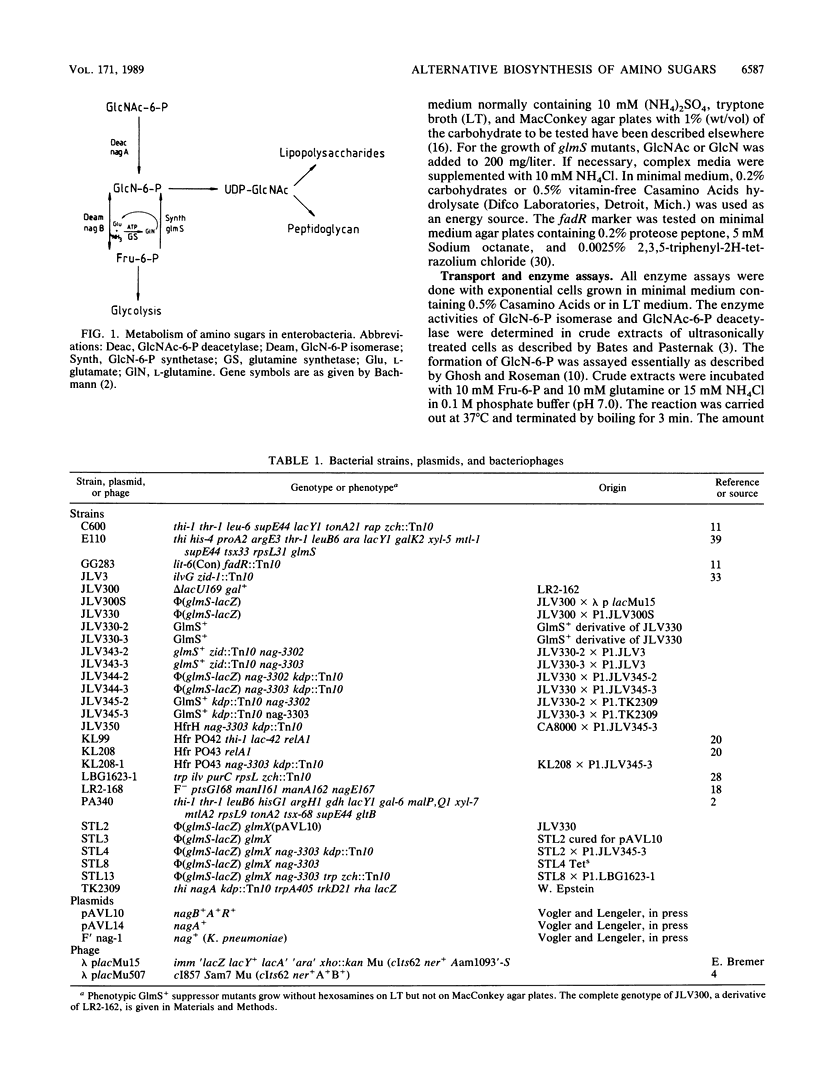
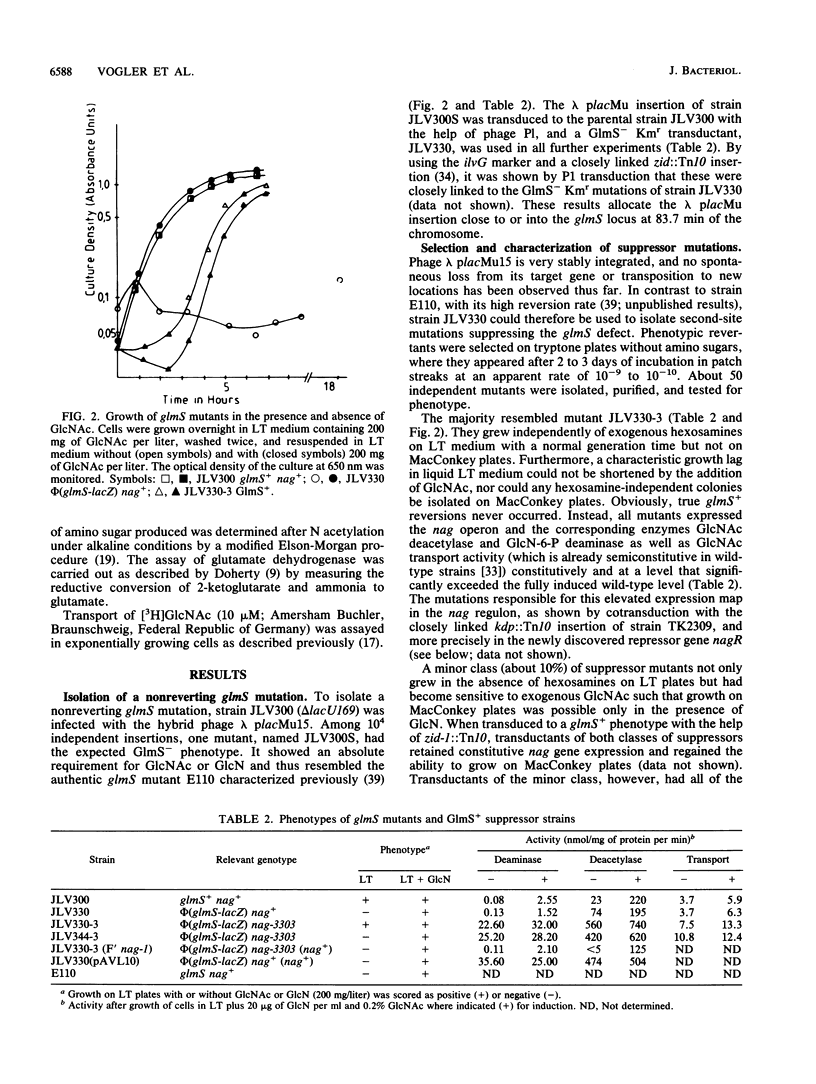
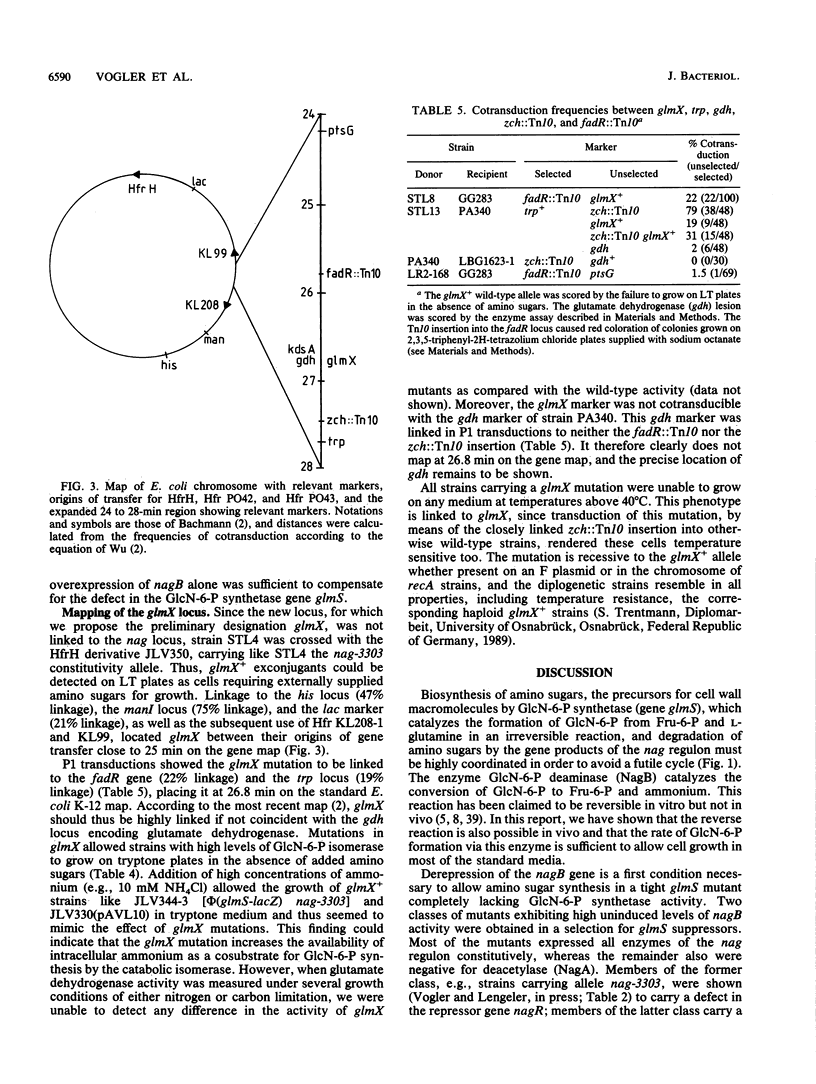
By inserting a lambda placMu bacteriophage into gene glmS encoding glucosamine 6-phosphate synthetase (GlmS), the key enzyme of amino sugar biosynthesis, a nonreverting mutant of Escherichia coli K-12 that was strictly dependent on exogenous N-acetyl-D-glucosamine or D-glucosamine was generated. Analysis of suppressor mutations rendering the mutant independent of amino sugar supply revealed that the catabolic enzyme D-glucosamine-6-phosphate isomerase (deaminase), encoded by gene nagB of the nag operon, was able to fulfill anabolic functions in amino sugar biosynthesis. The suppressor mutants invariably expressed the isomerase constitutively as a result of mutations in nagR, the locus for the repressor of the nag regulon. Suppression was also possible by transformation of glmS mutants with high-copy-number plasmids expressing the gene nagB. Efficient suppression of the glmS lesion, however, required mutations in a second locus, termed glmX, which has been localized to 26.8 min on the standard E. coli K-12 map. Its possible function in nitrogen or cell wall metabolism is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. S., Bulawa C. E., Raetz C. R. The biosynthesis of gram-negative endotoxin. Formation of lipid A precursors from UDP-GlcNAc in extracts of Escherichia coli. J Biol Chem. 1985 Dec 15;260(29):15536–15541. [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. FURTHER STUDIES ON THE REGULATION OF AMINO SUGAR METABOLISM IN BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:147–154. doi: 10.1042/bj0960147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weinstock G. M. Transposable lambda placMu bacteriophages for creating lacZ operon fusions and kanamycin resistance insertions in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1092–1099. doi: 10.1128/jb.162.3.1092-1099.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. Glucosamine metabolism. IV. Glucosamine-6-phosphate deaminase. J Biol Chem. 1958 Jun;232(2):807–827. [PubMed] [Google Scholar]
- COMB D. G., ROSEMAN S. Glucosamine-6-phosphate deaminase. Biochim Biophys Acta. 1956 Jul;21(1):193–194. doi: 10.1016/0006-3002(56)90124-x. [DOI] [PubMed] [Google Scholar]
- Calcagno M., Campos P. J., Mulliert G., Suástegui J. Purification, molecular and kinetic properties of glucosamine-6-phosphate isomerase (deaminase) from Escherichia coli. Biochim Biophys Acta. 1984 Jun 14;787(2):165–173. doi: 10.1016/0167-4838(84)90076-1. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Sanderson K. E., Ross H. Influence of temperature on growth of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium and Salmonella minnesota. Can J Microbiol. 1976 Oct;22(10):1540–1548. doi: 10.1139/m76-226. [DOI] [PubMed] [Google Scholar]
- Guarneros G., Machado G., Guzmán P., Garay E. Genetic and physical location of the Escherichia coli rap locus, which is essential for growth of bacteriophage lambda. J Bacteriol. 1987 Nov;169(11):5188–5192. doi: 10.1128/jb.169.11.5188-5192.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. P., Russell R. R. Mutations affecting amino sugar metabolism in Escherichia coli K-12. J Bacteriol. 1972 Jul;111(1):290–291. doi: 10.1128/jb.111.1.290-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada A., Nozaki Y., Kawashima F., Yoneda M. Regulation of glucosamine utilization in Staphylococcus aureus and Escherichia coli. J Gen Microbiol. 1977 Jun;100(2):329–337. doi: 10.1099/00221287-100-2-329. [DOI] [PubMed] [Google Scholar]
- Jayakumar A., Hwang S. J., Fabiny J. M., Chinault A. C., Barnes E. M., Jr Isolation of an ammonium or methylammonium ion transport mutant of Escherichia coli and complementation by the cloned gene. J Bacteriol. 1989 Feb;171(2):996–1001. doi: 10.1128/jb.171.2.996-1001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Amino-sugar transport systems of Escherichia coli K12. J Gen Microbiol. 1980 Apr;117(2):369–376. doi: 10.1099/00221287-117-2-369. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J., Auburger A. M., Mayer R., Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K 12. Mol Gen Genet. 1981;183(1):163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Characterisation of mutants of Escherichia coli K12, selected by resistance to streptozotocin. Mol Gen Genet. 1980;179(1):49–54. doi: 10.1007/BF00268445. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midelfort C. F., Rose I. A. Studies on the mechanism of Escherichia coli glucosamine-6-phosphate isomerase. Biochemistry. 1977 Apr 19;16(8):1590–1596. doi: 10.1021/bi00627a010. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Bulawa C. E., Raetz C. R. Two interacting mutations causing temperature-sensitive phosphatidylglycerol synthesis in Escherichia coli membranes. J Bacteriol. 1981 Jan;145(1):113–121. doi: 10.1128/jb.145.1.113-121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. Organisation of the Escherichia coli chromosome between genes glnS and glnU, V. Mol Gen Genet. 1987 Oct;209(3):618–620. doi: 10.1007/BF00331173. [DOI] [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Isolation of a mutant of Salmonella typhimurium dependent on D-arabinose-5-phosphate for growth and synthesis of 3-deoxy-D-mannoctulosonate (ketodeoxyoctonate). Proc Natl Acad Sci U S A. 1972 Dec;69(12):3756–3760. doi: 10.1073/pnas.69.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusina O. Iu, Il'ina T. S., Gershanovich V. N. Kartirovanie mutatsii vnutri genov, kodiruiushchikh ferment I i belok Hpr fosfoenolpiruvatzavisimoi fosfotransferaznoi sistemy u Escherichia coli K-12. Soobshchenie I. Kartirovanie mutatsii vnutri gena ptsI. Genetika. 1981;17(10):1771–1783. [PubMed] [Google Scholar]
- Sarvas M. Mutant of Escherichia coli K-12 defective in D-glucosamine biosynthesis. J Bacteriol. 1971 Feb;105(2):467–471. doi: 10.1128/jb.105.2.467-471.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Egan P. A., Chute H. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in gene fadR. J Bacteriol. 1980 May;142(2):621–632. doi: 10.1128/jb.142.2.621-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P. Complete structure of lipid A obtained from the lipopolysaccharides of the heptoseless mutant of Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12801–12803. [PubMed] [Google Scholar]
- Vogler A. P., Broekhuizen C. P., Schuitema A., Lengeler J. W., Postma P. W. Suppression of IIIGlc-defects by enzymes IINag and IIBgl of the PEP:carbohydrate phosphotransferase system. Mol Microbiol. 1988 Nov;2(6):719–726. doi: 10.1111/j.1365-2958.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Vogler A. P., Lengeler J. W. Indirect role of adenylate cyclase and cyclic AMP in chemotaxis to phosphotransferase system carbohydrates in Escherichia coli K-12. J Bacteriol. 1987 Feb;169(2):593–599. doi: 10.1128/jb.169.2.593-599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Gay N. J., Saraste M., Eberle A. N. DNA sequence around the Escherichia coli unc operon. Completion of the sequence of a 17 kilobase segment containing asnA, oriC, unc, glmS and phoS. Biochem J. 1984 Dec 15;224(3):799–815. doi: 10.1042/bj2240799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. The role of the phosphoenolpyruvate phosphotransferase system in the transport of N-acetyl-D-glucosamine by Escherichia coli. Biochem J. 1970 Jun;118(1):89–92. doi: 10.1042/bj1180089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschläger M., Hödl-Neuhofer A., Högenauer G. Localization of the kdsA gene with the aid of the physical map of the Escherichia coli chromosome. J Bacteriol. 1988 Nov;170(11):5382–5384. doi: 10.1128/jb.170.11.5382-5384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Wu T. C. Isolation and characterization of a glucosamine-requiring mutant of Escherichia coli K-12 defective in glucosamine-6-phosphate synthetase. J Bacteriol. 1971 Feb;105(2):455–466. doi: 10.1128/jb.105.2.455-466.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


