Abstract
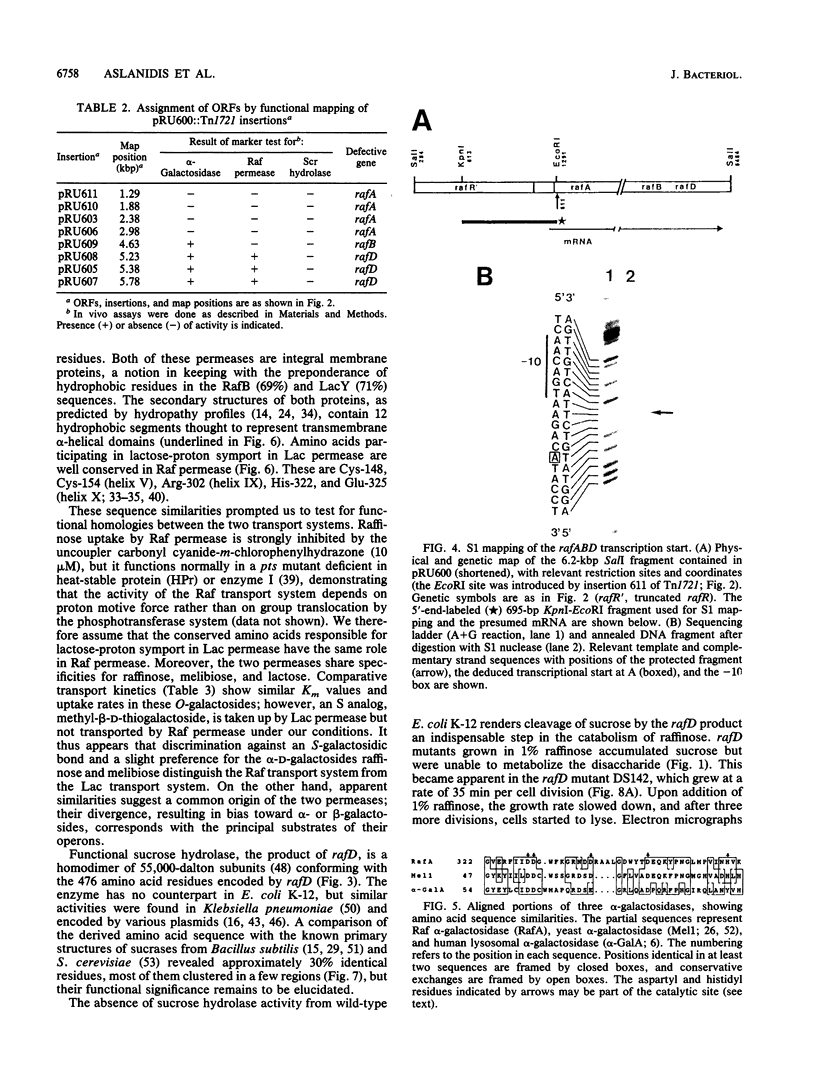
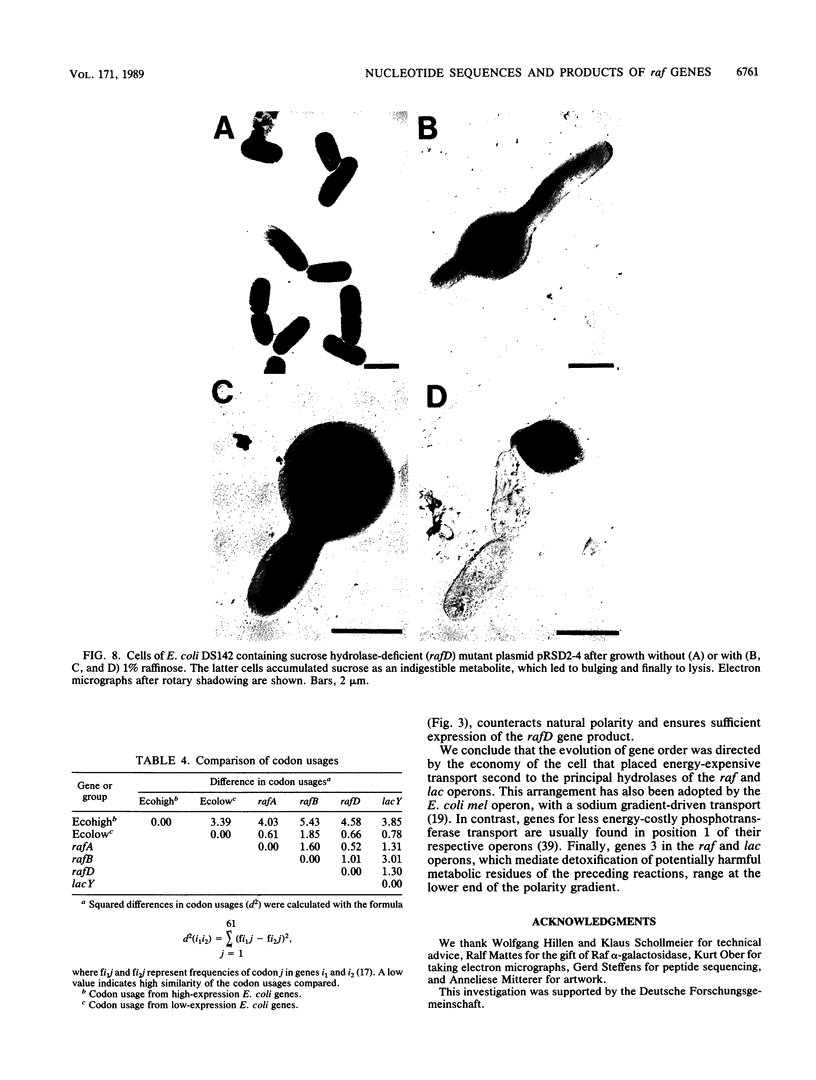
The plasmid-borne raf operon encodes functions required for inducible uptake and utilization of raffinose by Escherichia coli. Raf functions include active transport (Raf permease), alpha-galactosidase, and sucrose hydrolase, which are negatively controlled by the Raf repressor. We have defined the order and extent of the three structural genes, rafA, rafB, and rafD; these are contained in a 5,284-base-pair nucleotide sequence. By comparisons of derived primary structures with known subunit molecular weights and an N-terminal peptide sequence, rafA was assigned to alpha-galactosidase (708 amino acids), rafB was assigned to Raf permease (425 amino acids), and rafD was assigned to sucrose hydrolase (476 amino acids). Transcription was shown to initiate 13 nucleotides upstream of rafA; a putative promoter, a ribosome-binding site, and a transcription termination signal were identified. Striking similarities between Raf permease and lacY-encoded lactose permease, revealed by high sequence conservation (76%), overlapping substrate specificities, and similar transport kinetics, suggest a common origin of these transport systems. alpha-Galactosidase and sucrose hydrolase are not related to host enzymes but have their counterparts in other species. We propose a modular origin of the raf operon and discuss selective forces that favored the given gene organization also found in the E. coli lac operon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Altenbuchner J., Schmid K., Schmitt R. Tn1721-encoded tetracycline resistance: mapping of structural and regulatory genes mediating resistance. J Bacteriol. 1983 Jan;153(1):116–123. doi: 10.1128/jb.153.1.116-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews K. J., Lin E. C. Thiogalactoside transacetylase of the lactose operon as an enzyme for detoxification. J Bacteriol. 1976 Oct;128(1):510–513. doi: 10.1128/jb.128.1.510-513.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arps P. J., Marvel C. C., Rubin B. C., Tolan D. A., Penhoet E. E., Winkler M. E. Structural features of the hisT operon of Escherichia coli K-12. Nucleic Acids Res. 1985 Jul 25;13(14):5297–5315. doi: 10.1093/nar/13.14.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Calhoun D. H., Bernstein H. S., Hantzopoulos P., Quinn M., Desnick R. J. Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4859–4863. doi: 10.1073/pnas.83.13.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Burkardt H. J., Mattes R., Schmid K., Schmitt R. Properties of two conjugative plasmids mediating tetracycline resistance, raffinose catabolism and hydrogen sulfide production in Escherichia coli. Mol Gen Genet. 1978 Oct 25;166(1):75–84. doi: 10.1007/BF00379731. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Sommer H., Saedler H. Transposon Tn951 (TnLac) is defective and related to Tn3. Mol Gen Genet. 1981;184(2):241–248. doi: 10.1007/BF00272911. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P. M., Pridham J. B. Biochemistry of -galactosidases. Adv Enzymol Relat Areas Mol Biol. 1972;36:91–130. doi: 10.1002/9780470122815.ch3. [DOI] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Fouet A., Klier A., Rapoport G. Nucleotide sequence of the sucrase gene of Bacillus subtilis. Gene. 1986;45(2):221–225. doi: 10.1016/0378-1119(86)90258-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Evidence for Plasmid Linkage of Raffinose Utilization and Associated alpha-Galactosidase and Sucrose Hydrolase Activity in Pediococcus pentosaceus. Appl Environ Microbiol. 1986 Jan;51(1):105–109. doi: 10.1128/aem.51.1.105-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Hanatani M., Yazyu H., Shiota-Niiya S., Moriyama Y., Kanazawa H., Futai M., Tsuchiya T. Physical and genetic characterization of the melibiose operon and identification of the gene products in Escherichia coli. J Biol Chem. 1984 Feb 10;259(3):1807–1812. [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrchen M., Legler G. Identification of an essential carboxylate group at the active site of lacZ beta-galactosidase from Escherichia coli. Eur J Biochem. 1984 Feb 1;138(3):527–531. doi: 10.1111/j.1432-1033.1984.tb07947.x. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985 Jan;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Leder I. G., Perry J. W. Galactose stimulation of beta-galactosidase induction in galactokinaseless mutants of Escherichia coli. The induction of thiomethylgalactoside permease. J Biol Chem. 1967 Feb 10;242(3):457–462. [PubMed] [Google Scholar]
- Liljeström P. L., Liljeström P. Nucleotide sequence of the melA gene, coding for alpha-galactosidase in Escherichia coli K-12. Nucleic Acids Res. 1987 Mar 11;15(5):2213–2220. doi: 10.1093/nar/15.5.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P. L. The nucleotide sequence of the yeast MEL1 gene. Nucleic Acids Res. 1985 Oct 25;13(20):7257–7268. doi: 10.1093/nar/13.20.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I., Débarbouillé M., Ferrari E., Klier A., Rapoport G. Characterization of the levanase gene of Bacillus subtilis which shows homology to yeast invertase. Mol Gen Genet. 1987 Jun;208(1-2):177–184. doi: 10.1007/BF00330439. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Menick D. R., Carrasco N., Antes L., Patel L., Kaback H. R. lac permease of Escherichia coli: arginine-302 as a component of the postulated proton relay. Biochemistry. 1987 Oct 20;26(21):6638–6644. doi: 10.1021/bi00395a012. [DOI] [PubMed] [Google Scholar]
- Menick D. R., Sarkar H. K., Poonian M. S., Kaback H. R. cys154 Is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985 Oct 15;132(1):162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- Nagao Y., Nakada T., Imoto M., Shimamoto T., Sakai S., Tsuda M., Tsuchiya T. Purification and analysis of the structure of alpha-galactosidase from Escherichia coli. Biochem Biophys Res Commun. 1988 Feb 29;151(1):236–241. doi: 10.1016/0006-291x(88)90584-0. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Püttner I. B., Kaback H. R. lac permease of Escherichia coli containing a single histidine residue is fully functional. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1467–1471. doi: 10.1073/pnas.85.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowsky P., Schmitt R. Tn1721-encoded resolvase: structure of the tnpR gene and its in vitro functions. Mol Gen Genet. 1985;200(1):176–181. doi: 10.1007/BF00383332. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Ebner R., Altenbuchner J., Schmitt R., Lengeler J. W. Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400. Mol Microbiol. 1988 Jan;2(1):1–8. doi: 10.1111/j.1365-2958.1988.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Schmid K., Ritschewald S., Schmitt R. Relationships among raffinose plasmids determined by the immunochemical cross-reaction of their alpha-galactosidases. J Gen Microbiol. 1979 Oct;114(2):477–481. doi: 10.1099/00221287-114-2-477. [DOI] [PubMed] [Google Scholar]
- Schmid K., Schmitt R. Raffinose metabolism in Escherichia coli K12. Purification and properties of a new alpha-galactosidase specified by a transmissible plasmid. Eur J Biochem. 1976 Aug 1;67(1):95–104. doi: 10.1111/j.1432-1033.1976.tb10637.x. [DOI] [PubMed] [Google Scholar]
- Schmid K., Schupfner M., Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982 Jul;151(1):68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R. Analysis of melibiose mutants deficient in alpha-galactosidase and thiomethylgalactoside permease II in Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):462–471. doi: 10.1128/jb.96.2.462-471.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger G. A., Lengeler J. W. Analysis of sucrose catabolism in Klebsiella pneumoniae and in Scr+ derivatives of Escherichia coli K12. J Gen Microbiol. 1988 Jun;134(6):1635–1644. doi: 10.1099/00221287-134-6-1635. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Le Coq D., Aymerich S., Gonzy-Tréboul G., Gay P. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet. 1985;200(2):220–228. doi: 10.1007/BF00425427. [DOI] [PubMed] [Google Scholar]
- Sumner-Smith M., Bozzato R. P., Skipper N., Davies R. W., Hopper J. E. Analysis of the inducible MEL1 gene of Saccharomyces carlsbergensis and its secreted product, alpha-galactosidase (melibiase). Gene. 1985;36(3):333–340. doi: 10.1016/0378-1119(85)90188-x. [DOI] [PubMed] [Google Scholar]
- Taussig R., Carlson M. Nucleotide sequence of the yeast SUC2 gene for invertase. Nucleic Acids Res. 1983 Mar 25;11(6):1943–1954. doi: 10.1093/nar/11.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Kobayashi Y., Matsumoto H., Kamio Y. Molecular cloning and mapping of a deletion derivative of the plasmid Rts 1. Plasmid. 1981 Sep;6(2):222–234. doi: 10.1016/0147-619x(81)90068-8. [DOI] [PubMed] [Google Scholar]
- Ubben D., Schmitt R. Tn1721 derivatives for transposon mutagenesis, restriction mapping and nucleotide sequence analysis. Gene. 1986;41(2-3):145–152. doi: 10.1016/0378-1119(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]