Abstract
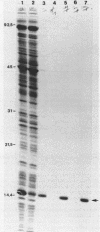
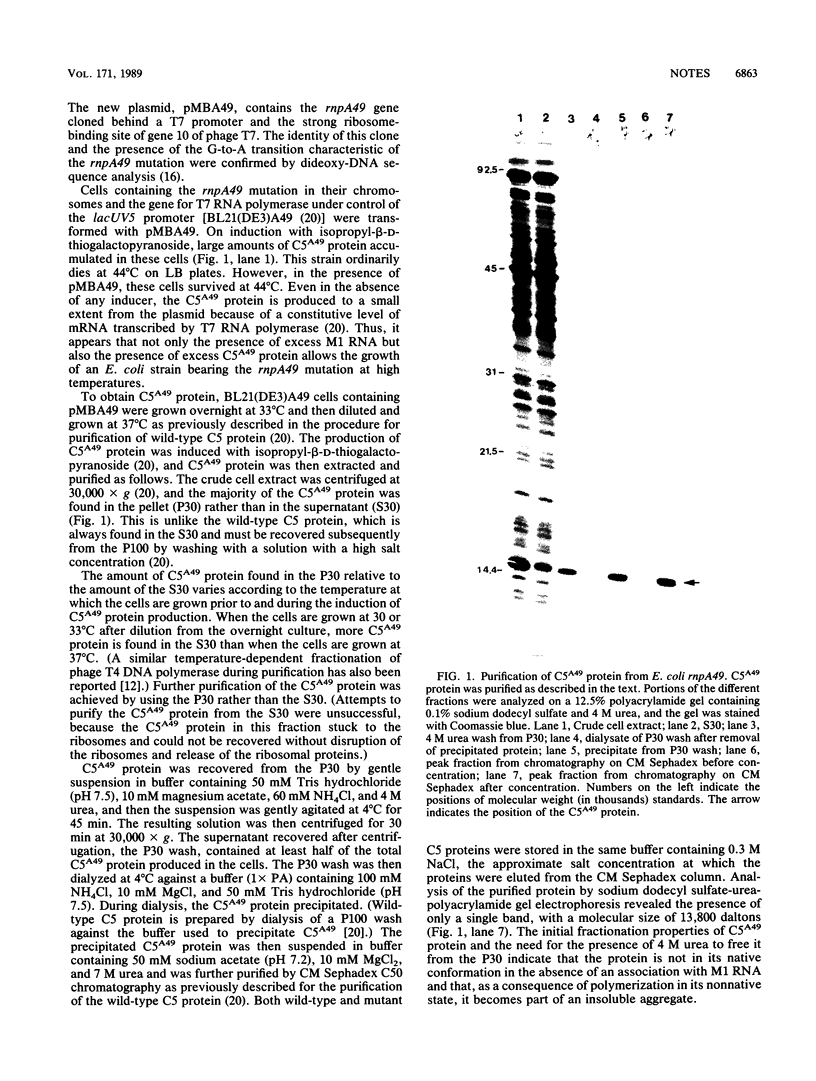
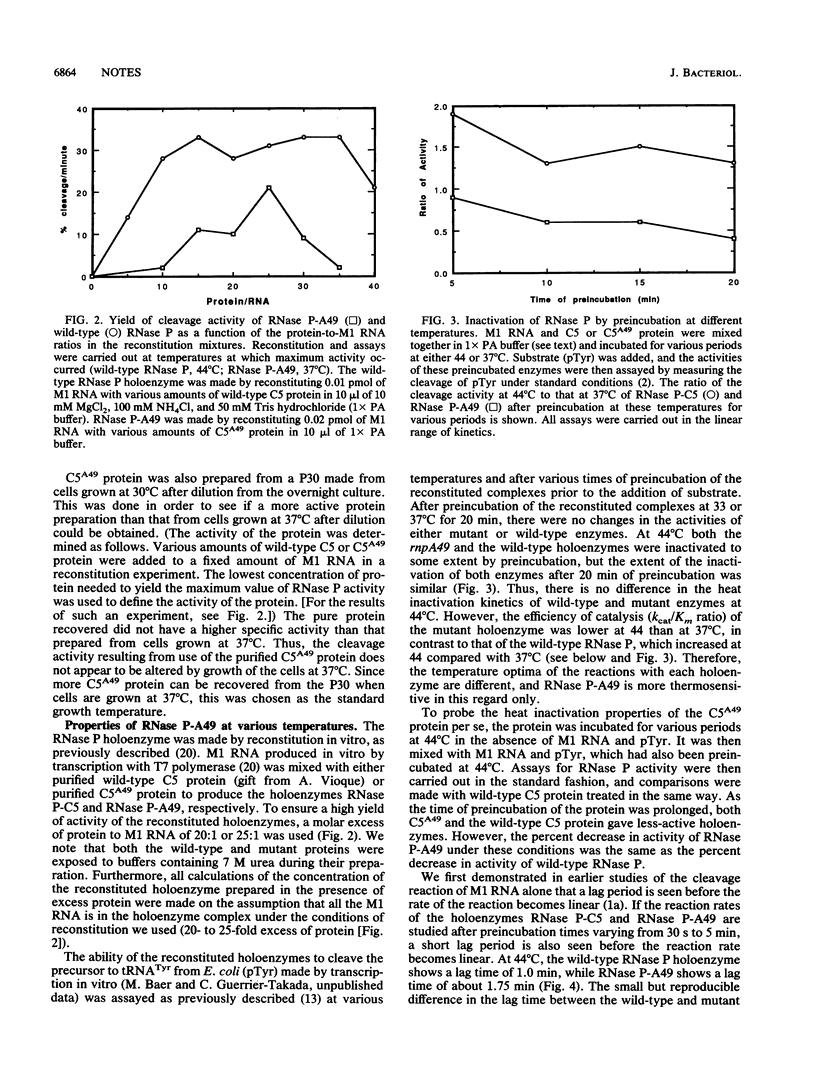
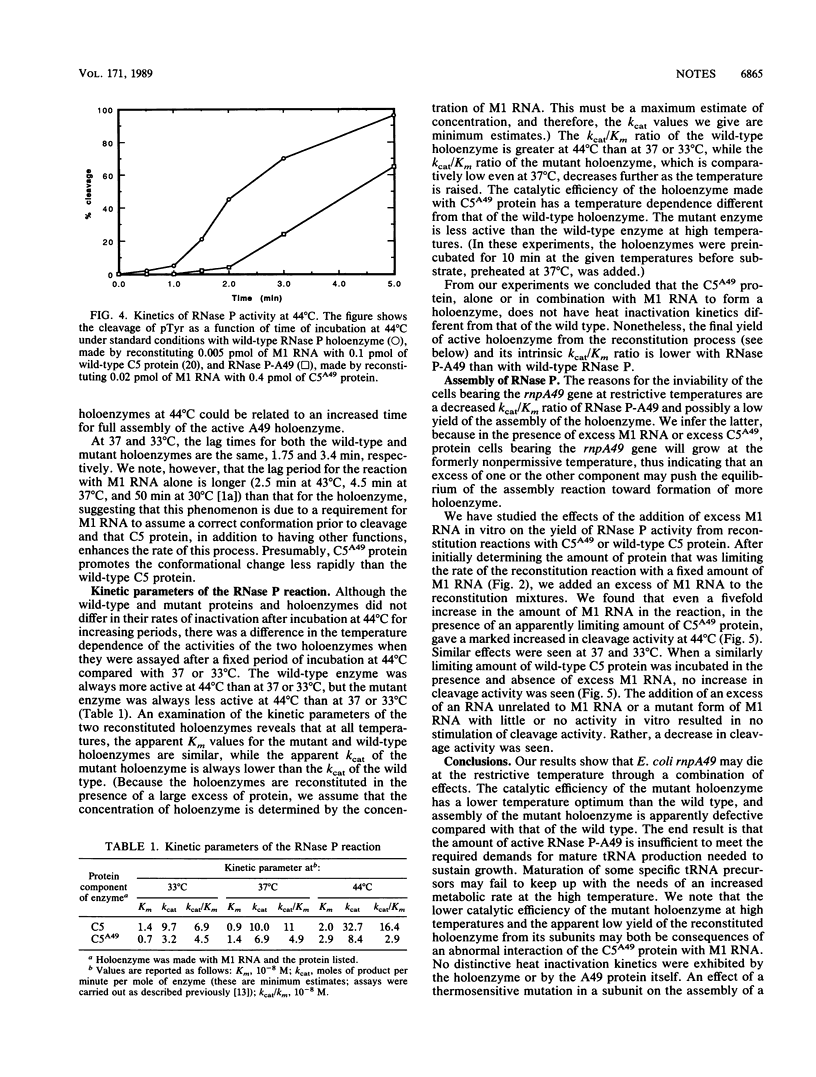
We have studied the assembly of Escherichia coli RNase P from its catalytic RNA subunit (M1 RNA) and its protein subunit (C5 protein). A mutant form of the protein subunit, C5A49, has been purified to apparent homogeneity from a strain of E. coli carrying a thermosensitive mutation in the rnpA gene. The heat inactivation kinetics of both wild-type and mutant holoenzymes are similar, an indication of equivalent thermal stability. However, when the catalytic efficiencies of the holoenzymes were compared, we found that the holoenzyme containing the mutant protein had a lower efficiency of cleavage than the wild-type holoenzyme at 33, 37, and 44 degrees C. We then explored the interaction of M1 RNA and C5 protein during the assembly of the holoenzyme. The yield of active holoenzyme obtained by reconstitution with wild-type M1 RNA and C5A49 protein in vitro can be considerably enhanced by the addition of excess M1 RNA, just as it can be in vivo. We concluded that the Arg-46----His-46 mutation in the C5A49 protein affects the ability of the protein to participate with M1 RNA in the normal assembly process of RNase P.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Guerrier-Takada C. M1 RNA, the RNA subunit of Escherichia coli ribonuclease P, can undergo a pH-sensitive conformational change. Biochemistry. 1986 Mar 25;25(6):1205–1208. doi: 10.1021/bi00354a002. [DOI] [PubMed] [Google Scholar]
- Altman S. Ribonuclease P: an enzyme with a catalytic RNA subunit. Adv Enzymol Relat Areas Mol Biol. 1989;62:1–36. doi: 10.1002/9780470123089.ch1. [DOI] [PubMed] [Google Scholar]
- Baer M. F., Reilly R. M., McCorkle G. M., Hai T. Y., Altman S., RajBhandary U. L. The recognition by RNase P of precursor tRNAs. J Biol Chem. 1988 Feb 15;263(5):2344–2351. [PubMed] [Google Scholar]
- Goldenberg D. P., King J. Temperature-sensitive mutants blocked in the folding or subunit of the bacteriophage P22 tail spike protein. II. Active mutant proteins matured at 30 degrees C. J Mol Biol. 1981 Feb 5;145(4):633–651. doi: 10.1016/0022-2836(81)90307-7. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. P., Smith D. H., King J. Genetic analysis of the folding pathway for the tail spike protein of phage P22. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7060–7064. doi: 10.1073/pnas.80.23.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Scholla C. A. Asymmetric maturation of a dimeric transfer RNA precursor. J Mol Biol. 1980 May 25;139(3):349–375. doi: 10.1016/0022-2836(80)90135-7. [DOI] [PubMed] [Google Scholar]
- Hansen F. G., Hansen E. B., Atlung T. Physical mapping and nucleotide sequence of the rnpA gene that encodes the protein component of ribonuclease P in Escherichia coli. Gene. 1985;38(1-3):85–93. doi: 10.1016/0378-1119(85)90206-9. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Gurevitz M., Apirion D. A small RNA that complements mutants in the RNA processing enzyme ribonuclease P. J Mol Biol. 1982 Dec 15;162(3):515–533. doi: 10.1016/0022-2836(82)90386-2. [DOI] [PubMed] [Google Scholar]
- Kirsebom L. A., Baer M. F., Altman S. Differential effects of mutations in the protein and RNA moieties of RNase P on the efficiency of suppression by various tRNA suppressors. J Mol Biol. 1988 Dec 20;204(4):879–888. doi: 10.1016/0022-2836(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Kole R., Baer M. F., Stark B. C., Altman S. E. coli RNAase P has a required RNA component. Cell. 1980 Apr;19(4):881–887. doi: 10.1016/0092-8674(80)90079-3. [DOI] [PubMed] [Google Scholar]
- Lawrence N. P., Altman S. Site-directed mutagenesis of M1 RNA, the RNA subunit of Escherichia coli ribonuclease P. The effects of an addition and small deletions on catalytic function. J Mol Biol. 1986 Sep 20;191(2):163–175. doi: 10.1016/0022-2836(86)90253-6. [DOI] [PubMed] [Google Scholar]
- Lin T. C., Rush J., Spicer E. K., Konigsberg W. H. Cloning and expression of T4 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7000–7004. doi: 10.1073/pnas.84.20.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain W. H., Guerrier-Takada C., Altman S. Model substrates for an RNA enzyme. Science. 1987 Oct 23;238(4826):527–530. doi: 10.1126/science.2443980. [DOI] [PubMed] [Google Scholar]
- Motamedi H., Lee K., Nichols L., Schmidt F. J. An RNA species involved in Escherichia coli ribonuclease P activity. Gene cloning and effect on transfer RnA synthesis in vivo. J Mol Biol. 1982 Dec 15;162(3):535–550. doi: 10.1016/0022-2836(82)90387-4. [DOI] [PubMed] [Google Scholar]
- Sakano H., Yamada S., Ikemura T., Shimura Y., Ozeki H. Temperature sensitive mutants of Escherichia coli for tRNA synthesis. Nucleic Acids Res. 1974 Mar;1(3):355–371. doi: 10.1093/nar/1.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Primakoff P. Mutants of Escherichia coli thermosensitive for the synthesis of transfer RNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2091–2095. doi: 10.1073/pnas.70.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedl P., Primakoff P., Roberts J. Processing of E. coli tRNA precursors. Brookhaven Symp Biol. 1975 Jul;(26):53–76. [PubMed] [Google Scholar]
- Smith D. H., King J. Temperature-sensitive mutants blocked in the folding or subunit assembly of the bacteriophage P22 tail spike protein. III. Intensive polypeptide chains synthesized at 39 degrees C. J Mol Biol. 1981 Feb 5;145(4):653–676. doi: 10.1016/0022-2836(81)90308-9. [DOI] [PubMed] [Google Scholar]
- Vioque A., Arnez J., Altman S. Protein-RNA interactions in the RNase P holoenzyme from Escherichia coli. J Mol Biol. 1988 Aug 20;202(4):835–848. doi: 10.1016/0022-2836(88)90562-1. [DOI] [PubMed] [Google Scholar]