Abstract
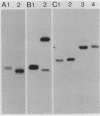
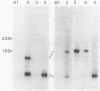
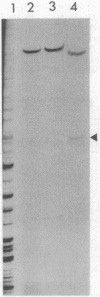
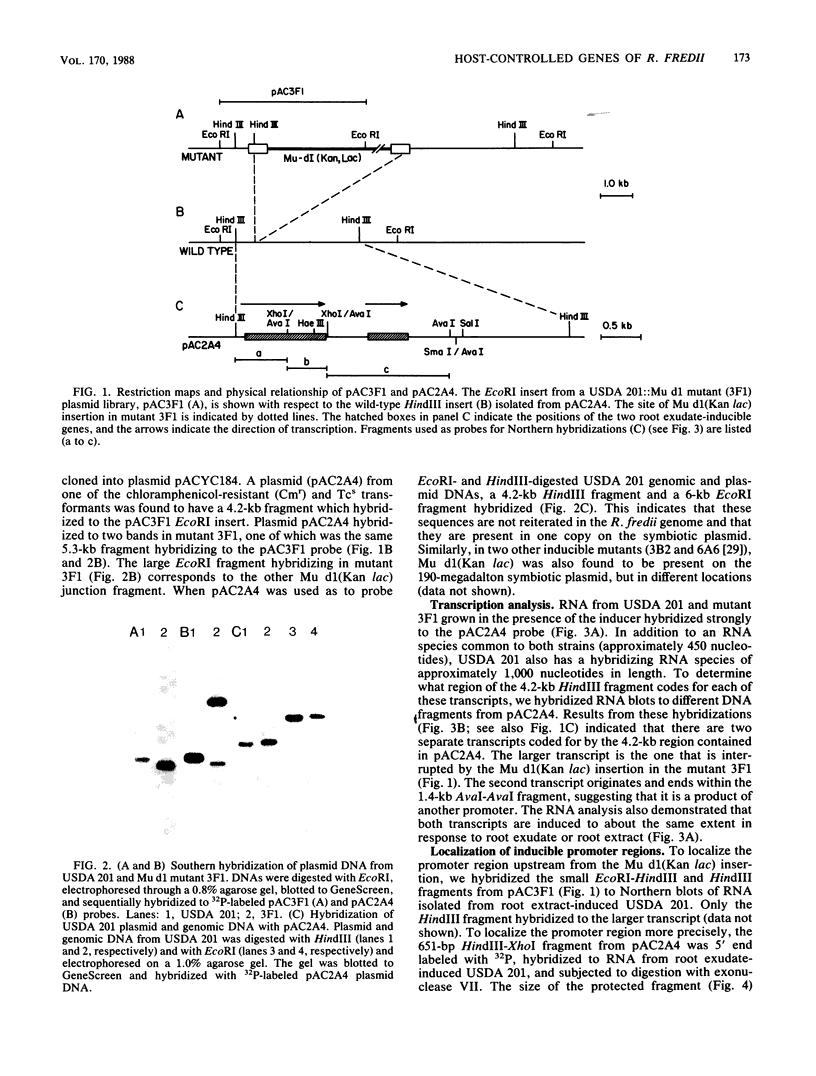
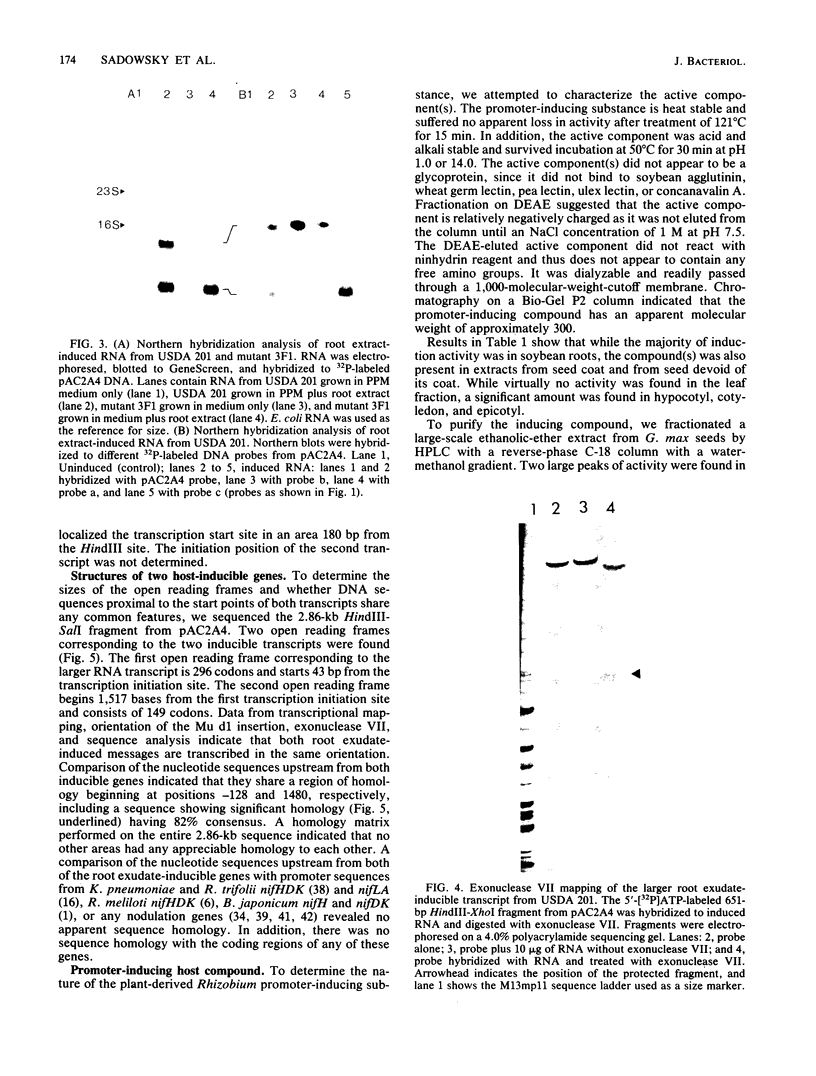
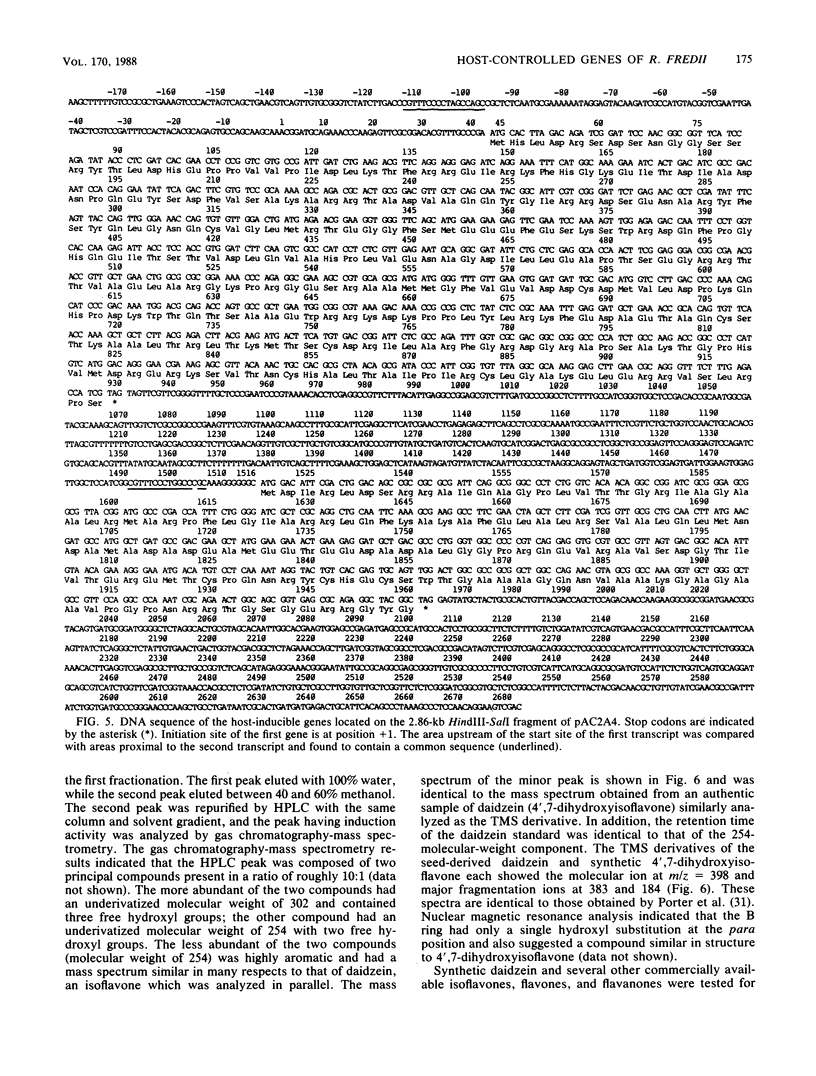
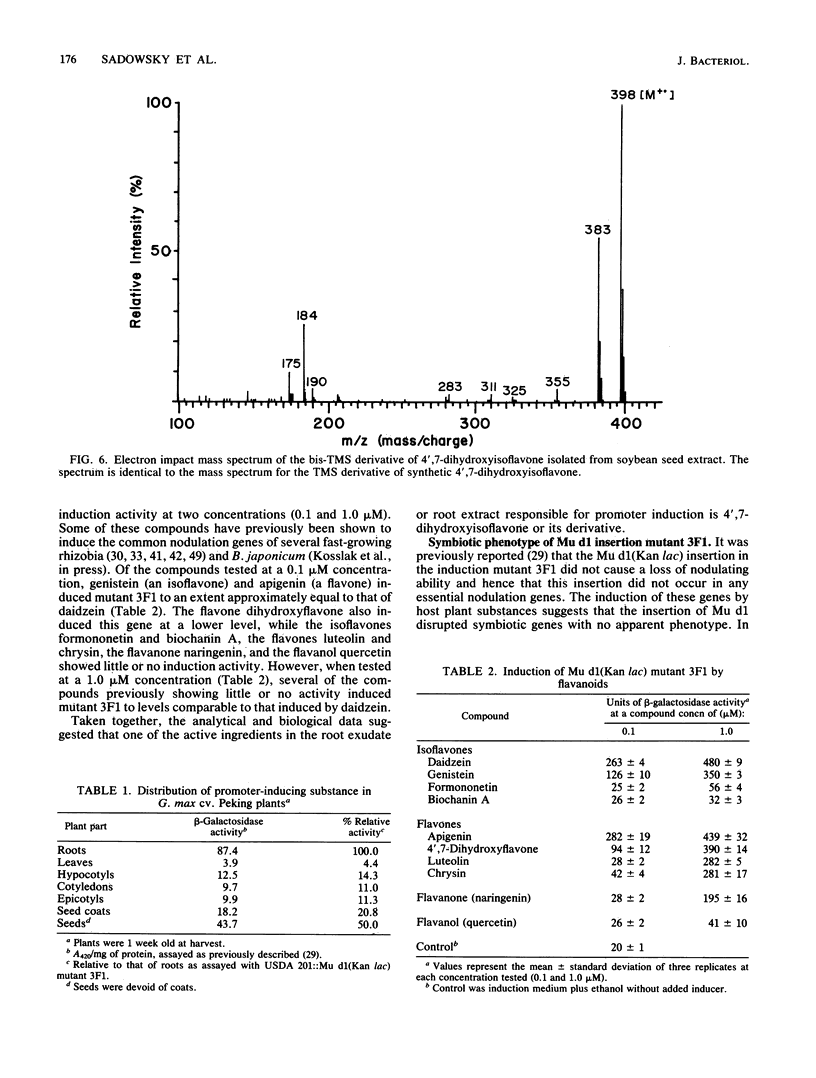
Random transcription fusions with Mu d1(Kan lac) generated three mutants in Rhizobium fredii (strain USDA 201) which showed induction of beta-galactosidase when grown in root exudate of the host plants Glycine max, Phaseolus vulgaris, and Vigna ungliculata. Two genes were isolated from a library of total plasmid DNA of one of the mutants, 3F1. These genes, present in tandem on a 4.2-kilobase HindIII fragment, appear in one copy each on the symbiotic plasmid and do not hybridize to the Rhizobium meliloti common nodulation region. They comprise two separate transcriptional units coding for about 450 and 950 nucleotides, both of which are transcribed in the same direction. The two open reading frames are separated by 586 base pairs, and the 5H regions of the two genes show a common sequence. No similarity was found with the promoter areas of Rhizobium trifolii, R. meliloti, or Bradyrhizobium japonicum nif genes and with any known nodulation genes. Regions homologous to both sequences were detected in EcoRI digests of genomic DNAs from B. japonicum USDA 110, USDA 122, and 61A76, but not in genomic DNA from R. trifolii, Rhizobium leguminosarum, or Rhizobium phaseoli. Mass spectrometry and nuclear magnetic resonance analysis indicated that the inducing compound has properties of 4',7-dihydroxyisoflavone, daidzein. These results suggest that, in addition to common nodulation genes, several other genes appear to be specifically induced by compounds in the root exudate of the host plants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. H., Chelm B. K. The nifH and nifDK promoter regions from Rhizobium japonicum share structural homologies with each other and with nitrogen-regulated promoters from other organisms. J Mol Appl Genet. 1984;2(4):392–405. [PubMed] [Google Scholar]
- Ayanaba A., Haugland R. A., Sadowsky M. J., Upchurch R. G., Weiland K. D., Zablotowicz R. M. Rapid Colored-Nodule Assay for Assessing Root Exudate-Enhanced Competitiveness of Bradyrhizobium japonicum. Appl Environ Microbiol. 1986 Oct;52(4):847–851. doi: 10.1128/aem.52.4.847-851.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Better M., Lewis B., Corbin D., Ditta G., Helinski D. R. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell. 1983 Dec;35(2 Pt 1):479–485. doi: 10.1016/0092-8674(83)90181-2. [DOI] [PubMed] [Google Scholar]
- Bhagwat A. A., Thomas J. Legume-Rhizobium interactions: cowpea root exudate elicits faster nodulation response by Rhizobium species. Appl Environ Microbiol. 1982 Apr;43(4):800–805. doi: 10.1128/aem.43.4.800-805.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: III. Influence of Rhizosphere/Rhizoplane Culture Conditions on the Soybean Lectin-binding Properties of Rhizobia. Plant Physiol. 1978 Jul;62(1):71–74. doi: 10.1104/pp.62.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseling T., Been C., Klugkist J., Kammen A., Nadler K. Nodule-specific host proteins in effective and ineffective root nodules of Pisum sativum. EMBO J. 1983;2(6):961–966. doi: 10.1002/j.1460-2075.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Whitney P., Magasanik B. Reaction of lac-specific ribonucleic acid from Escherichia coli with lac deoxyribonucleic acid. J Biol Chem. 1974 Oct 25;249(20):6548–6555. [PubMed] [Google Scholar]
- Currier W. W., Strobel G. A. Chemotaxis of Rhizobium spp. to Plant Root Exudates. Plant Physiol. 1976 May;57(5):820–823. doi: 10.1104/pp.57.5.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier W. W., Strobel G. A. Chemotaxis of Rhizobium spp. to a Glycoprotein Produced by Birdsfoot Trefoil Roots. Science. 1977 Apr 22;196(4288):434–436. doi: 10.1126/science.196.4288.434. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Drummond M., Clements J., Merrick M., Dixon R. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):302–307. doi: 10.1038/301302a0. [DOI] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Host recognition in the Rhizobium-soybean symbiosis: detection of a protein factor in soybean root exudate which is involved in the nodulation process. Plant Physiol. 1984 Jan;74(1):84–89. doi: 10.1104/pp.74.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R., Verma D. P. Interspecific plasmid and genomic DNA sequence homologies and localization of nif genes in effective and ineffective strains of Rhizobium japonicum. J Mol Appl Genet. 1981;1(3):205–217. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B., Dowdle S., Sadowsky M. J. Competition of Rhizobium japonicum Strains in Early Stages of Soybean Nodulation. Appl Environ Microbiol. 1983 Oct;46(4):870–873. doi: 10.1128/aem.46.4.870-873.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Rossen L., Shearman C. A., Johnston A. W., Downie J. A. The nodD gene of Rhizobium leguminosarum is autoregulatory and in the presence of plant exudate induces the nodA,B,C genes. EMBO J. 1985 Dec 16;4(13A):3369–3373. doi: 10.1002/j.1460-2075.1985.tb04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostas K., Kondorosi E., Horvath B., Simoncsits A., Kondorosi A. Conservation of extended promoter regions of nodulation genes in Rhizobium. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1757–1761. doi: 10.1073/pnas.83.6.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Bohlool B. B. Possible involvement of a megaplasmid in nodulation of soybeans by fast-growing rhizobia from china. Appl Environ Microbiol. 1983 Oct;46(4):906–911. doi: 10.1128/aem.46.4.906-911.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L. Initiation of plant root-microbe interactions. Annu Rev Microbiol. 1979;33:355–376. doi: 10.1146/annurev.mi.33.100179.002035. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Watson J. M. Conservation of nif- and species-specific domains within repeated promoter sequences from fast-growing Rhizobium species. Nucleic Acids Res. 1985 May 24;13(10):3407–3418. doi: 10.1093/nar/13.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. F. Conserved nodulation genes from the non-legume symbiont Bradyrhizobium sp. (Parasponia). Nucleic Acids Res. 1986 Apr 11;14(7):2905–2919. doi: 10.1093/nar/14.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Biological nitrogen fixation: primary structure of the Klebsiella pneumoniae nifH and nifD genes. J Mol Appl Genet. 1981;1(1):71–81. [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brussel A. A., Zaat S. A., Cremers H. C., Wijffelman C. A., Pees E., Tak T., Lugtenberg B. J. Role of plant root exudate and Sym plasmid-localized nodulation genes in the synthesis by Rhizobium leguminosarum of Tsr factor, which causes thick and short roots on common vetch. J Bacteriol. 1986 Feb;165(2):517–522. doi: 10.1128/jb.165.2.517-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Spaink H. P., van Brussel A. A., Okker R. J., Lugtenberg B. J. Induction of the nodA promoter of Rhizobium leguminosarum Sym plasmid pRL1JI by plant flavanones and flavones. J Bacteriol. 1987 Jan;169(1):198–204. doi: 10.1128/jb.169.1.198-204.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]