Abstract
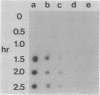
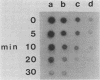
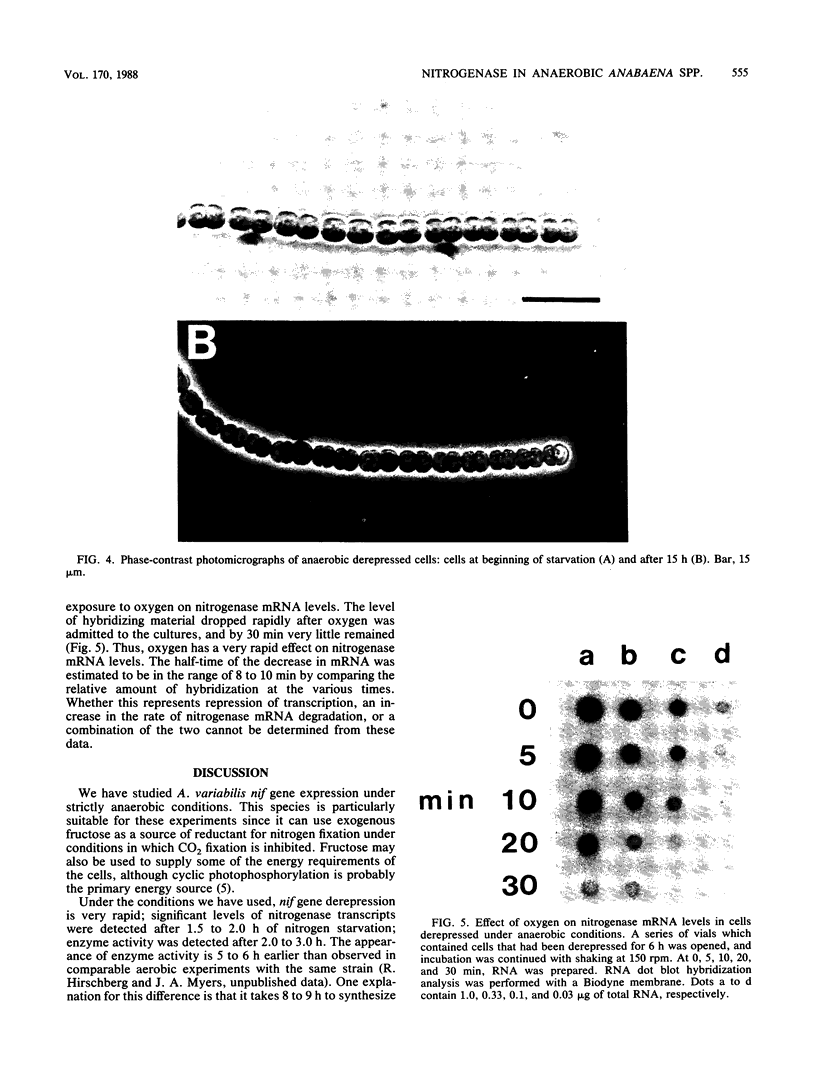
Derepression of nitrogenase gene expression was studied at the mRNA and enzyme activity levels in anaerobic cultures of Anabaena variabilis 29413. Cells, previously grown with ammonium chloride, were incubated in the absence of fixed nitrogen compounds under an Ar atmosphere with dichlorophenyldimethyl-urea present to inhibit oxygen evolution. The appearance of nitrogenase mRNA (measured by dot blot hybridization analysis) and nitrogenase activity (measured as acetylene-reducing activity) was followed, and the cells were also observed by phase-contrast microscopy. Nitrogenase mRNA could be detected after 1.5 to 2.0 h of nitrogen starvation; enzyme activity appeared about 1 h later. Although enzyme activity increased for many hours, mRNA levels reached a steady state rapidly. Neither heterocysts nor proheterocysts formed under these conditions; however, the cells were observed to shrink and become chlorotic. When anaerobic, derepressed cultures were exposed to oxygen, nitrogenase mRNA levels decreased very rapidly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. B., Arnon D. I. Studies on Nitrogen-Fixing Blue-Green Algae. I. Growth and Nitrogen Fixation by Anabaena Cylindrica Lemm. Plant Physiol. 1955 Jul;30(4):366–372. doi: 10.1104/pp.30.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S., Carr N. G. Heterocyst and nitrogenase development in Anabaena cylindrica. J Gen Microbiol. 1976 Sep;96(1):175–184. doi: 10.1099/00221287-96-1-175. [DOI] [PubMed] [Google Scholar]
- Cannon M., Hill S., Kavanaugh E., Cannon F. A molecular genetic study of nif expression in Klebsiella pneumoniae at the level of transcription, translation and nitrogenase activity. Mol Gen Genet. 1985;198(2):198–206. doi: 10.1007/BF00382996. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins J. J., Brill W. J. Control of Klebsiella pneumoniae nif mRNA synthesis. J Bacteriol. 1985 Jun;162(3):1186–1190. doi: 10.1128/jb.162.3.1186-1190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. J., Roberts G. P., Brill W. J. Posttranscriptional control of Klebsiella pneumoniae nif mRNA stability by the nifL product. J Bacteriol. 1986 Oct;168(1):173–178. doi: 10.1128/jb.168.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H., Haselkorn R. The program of protein synthesis during heterocyst differentiation in nitrogen-fixing blue-green algae. Cell. 1974 Oct;3(2):169–170. doi: 10.1016/0092-8674(74)90121-4. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Rice D., Curtis S. E., Robinson S. J. Organization and transcription of genes important in Anabaena heterocyst differentiation. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):181–193. doi: 10.1016/s0769-2609(83)80104-5. [DOI] [PubMed] [Google Scholar]
- Kaluza K., Hennecke H. Regulation of nitrogenase messenger RNA synthesis and stability in Klebsiella pneumoniae. Arch Microbiol. 1981 Sep;130(1):38–43. doi: 10.1007/BF00527069. [DOI] [PubMed] [Google Scholar]
- Kong Q. T., Wu Q. L., Ma Z. F., Shen S. C. Oxygen sensitivity of the nifLA promoter of Klebsiella pneumoniae. J Bacteriol. 1986 Apr;166(1):353–356. doi: 10.1128/jb.166.1.353-356.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A. J., Hontelez J. G., Roozendaal B., van Kammen A. On the operon structure of the nitrogenase genes of Rhizobium leguminosarum and Azotobacter vinelandii. Nucleic Acids Res. 1982 Jul 24;10(14):4147–4157. doi: 10.1093/nar/10.14.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. In vitro protein synthesis and measurement of the stability of messenger RNA in the blue-green alga, anabaena variabilis. J Gen Microbiol. 1974 Mar;81(1):47–58. doi: 10.1099/00221287-81-1-47. [DOI] [PubMed] [Google Scholar]
- Murry M. A., Horne A. J., Benemann J. R. Physiological Studies of Oxygen Protection Mechanisms in the Heterocysts of Anabaena cylindrica. Appl Environ Microbiol. 1984 Mar;47(3):449–454. doi: 10.1128/aem.47.3.449-454.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Wolk C. P. High recovery of nitrogenase activity and of Fe-labeled nitrogenase in heterocysts isolated from Anabaena variabilis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6271–6275. doi: 10.1073/pnas.75.12.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Rogerson A. C. Modifiers of heterocyst repression and spacing and formation of heterocysts without nitrogenase in the cyanobacterium Anabaena variabilis. J Bacteriol. 1979 Oct;140(1):213–219. doi: 10.1128/jb.140.1.213-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. D. Some aspects of structure and function in N2-fixing cyanobacteria. Annu Rev Microbiol. 1980;34:497–536. doi: 10.1146/annurev.mi.34.100180.002433. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]