Abstract
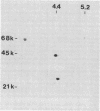
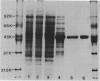
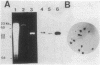
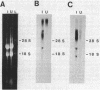
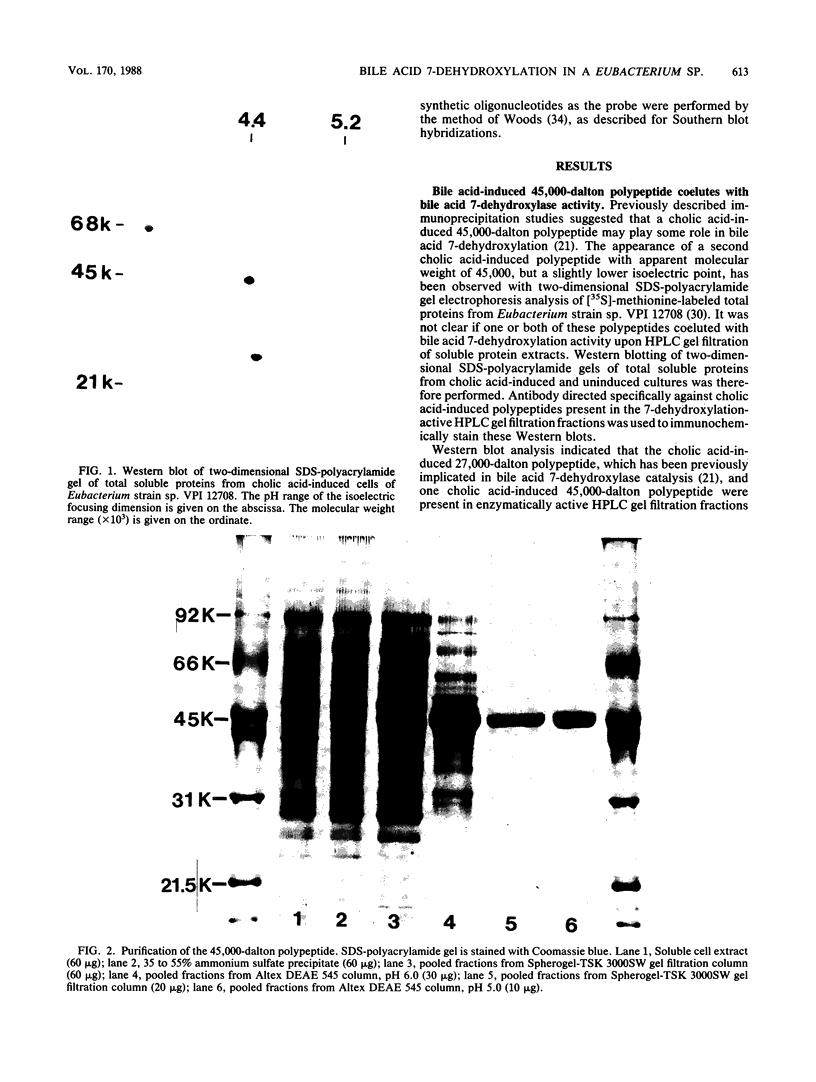
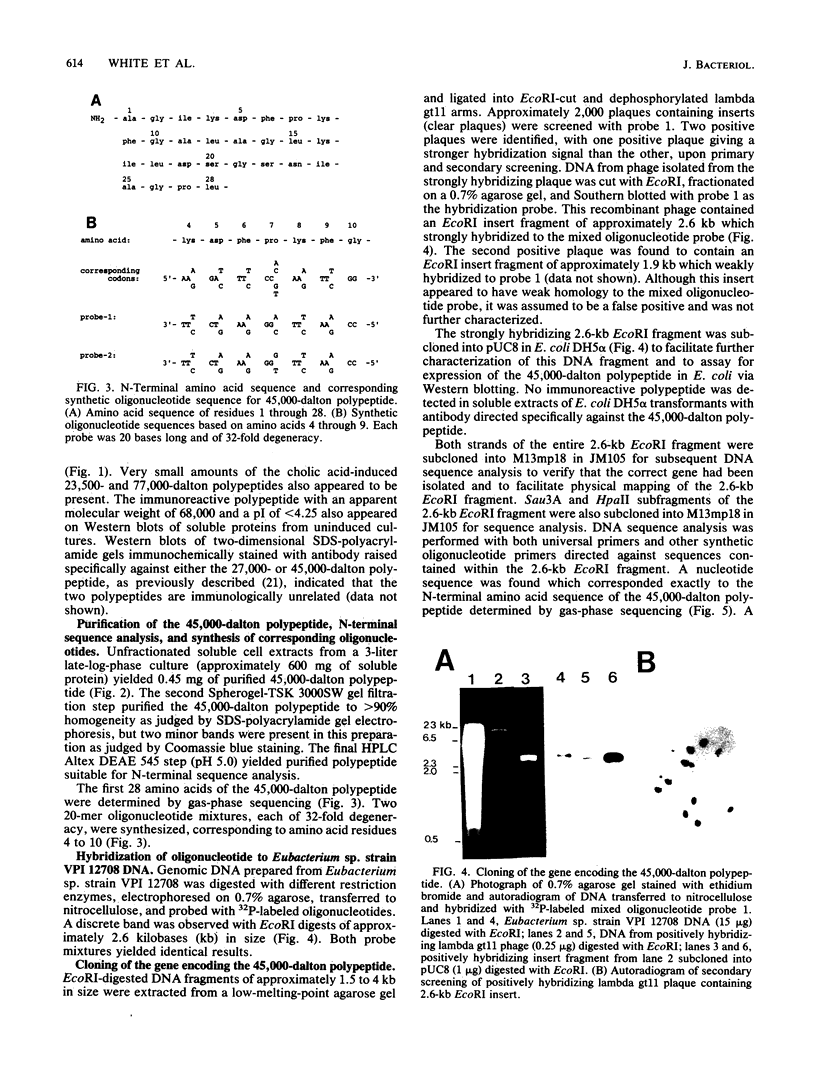
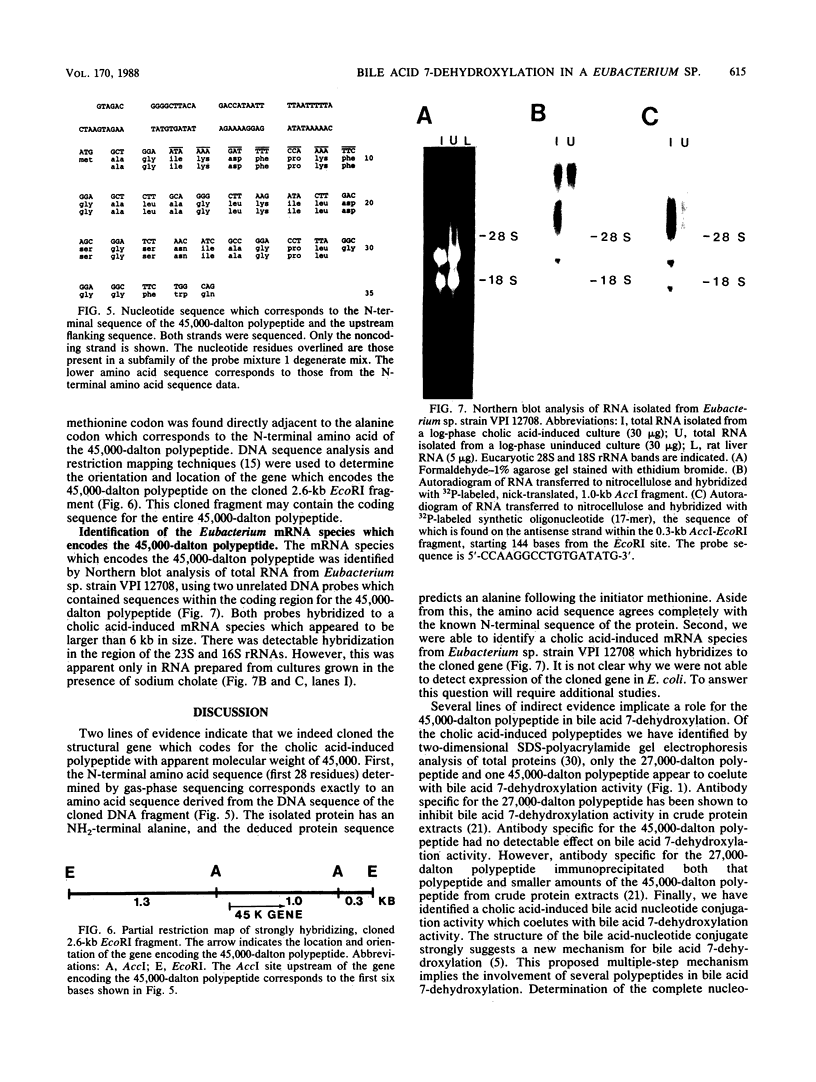
Eubacterium sp. strain VPI 12708 is an intestinal anaerobic bacterium which possesses an inducible bile acid 7-dehydroxylation activity. Two cholic acid-induced polypeptides with apparent molecular weights of 27,000 and 45,000, respectively, coeluted with bile acid 7-dehydroxylation activity upon anaerobic high-performance gel filtration chromatography of crude cellular protein extracts. The 45,000-dalton polypeptide was purified to greater than 95% homogeneity by high-performance liquid chromatography gel filtration and high-performance liquid-DEAE chromatography. The first 28 amino acid residues of the N terminus of this polypeptide were determined by gas-phase sequencing, and a corresponding mixed oligonucleotide (20-mer) was synthesized. Southern blot analysis of EcoRI total digests of chromosomal DNA showed a 2.6-kilobase fragment which hybridized to the 32P-labeled 20-mer. This fragment was enriched for by size fractionation of an EcoRI total digest of genomic DNA and ligated into bacteriophage lambda gt11. Recombinant phage containing the putative gene encoding the 45,000-dalton polypeptide were detected with the 32P-labeled 20-mer by plaque hybridization techniques. The insert was 2.6 kilobases in length and may contain the entire coding sequence for the 45,000-dalton polypeptide. The 2.6-kilobase insert was subcloned into pUC8 and transformed into Escherichia coli DH5 alpha. However, the 45,000-dalton polypeptide was not detected in cell extracts of this organism when specific antibody was used. Preliminary nucleic acid sequence data correlated exactly with the amino acid sequence. A cholic acid-induced mRNA species of greater than 6 kilobases in size was identified by Northern (RNA) blot analysis of total RNA, suggesting that the gene coding for this polypeptide is part of a larger operon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagheri S. A., Bolt M. G., Boyer J. L., Palmer R. H. Stimulation of thymidine incorporation in mouse liver and biliary tract epithelium by lithocholate and deoxycholate. Gastroenterology. 1978 Feb;74(2 Pt 1):188–192. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coleman J. P., White W. B., Egestad B., Sjövall J., Hylemon P. B. Biosynthesis of a novel bile acid nucleotide and mechanism of 7 alpha-dehydroxylation by an intestinal Eubacterium species. J Biol Chem. 1987 Apr 5;262(10):4701–4707. [PubMed] [Google Scholar]
- Coleman J. P., White W. B., Hylemon P. B. Molecular cloning of bile acid 7-dehydroxylase from Eubacterium sp. strain VPI 12708. J Bacteriol. 1987 Apr;169(4):1516–1521. doi: 10.1128/jb.169.4.1516-1521.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Kelsey M. I., Pienta R. J. Transformation of hamster embryo cells by cholesterol-alpha-epoxide and lithocholic acid. Cancer Lett. 1979 Mar;6(3):143–149. doi: 10.1016/s0304-3835(79)80025-7. [DOI] [PubMed] [Google Scholar]
- Kulkarni M. S., Heidepriem P. M., Yielding K. L. Production by lithocholic acid of DNA strand breaks in L1210 cells. Cancer Res. 1980 Aug;40(8 Pt 1):2666–2669. [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Liao H. H., Rabinowitz J. C. Clostridial apoferredoxin messenger ribonucleic acid. Assay and partial purification. Biochim Biophys Acta. 1980 Jul 29;608(2):301–314. doi: 10.1016/0005-2787(80)90176-8. [DOI] [PubMed] [Google Scholar]
- Lipsky R. H., Hylemon P. B. Characterization of a NADH:flavin oxidoreductase induced by cholic acid in a 7 alpha-dehydroxylating intestinal Eubacterium species. Biochim Biophys Acta. 1980 Apr 11;612(2):328–336. doi: 10.1016/0005-2744(80)90115-1. [DOI] [PubMed] [Google Scholar]
- Low-Beer T. S., Nutter S. Colonic bacterial activity, biliary cholesterol saturation, and pathogenesis of gallstones. Lancet. 1978 Nov 18;2(8099):1063–1065. doi: 10.1016/s0140-6736(78)91800-7. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Magadia N. E., Weisburger J. H., Wynder E. L. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974 Oct;53(4):1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- Owen R. W., Hill M. J., Bilton R. F. Biotransformation of chenodeoxycholic acid by Pseudomonas species NCIB 10590 under anaerobic conditions. J Lipid Res. 1983 Sep;24(9):1109–1118. [PubMed] [Google Scholar]
- Paone D. A., Hylemon P. B. HPLC purification and preparation of antibodies to cholic acid-inducible polypeptides from Eubacterium sp. V.P.I. 12708. J Lipid Res. 1984 Dec 1;25(12):1343–1349. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrede S., Björkhem I. Biosynthesis of cholestanol from intestinal 7 alpha-hydroxy-4-cholesten-3-one. J Biol Chem. 1982 Jul 25;257(14):8363–8367. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Cacciapuoti A. F., Fricke R. J., Whitehead T. R., Mosbach E. H., Hylemon P. B. Cofactor requiremets for 7 alpha-dehydroxylation of cholic and chenodeoxycholic acid in cell extracts of the intestinal anaerobic bacterium, Eubacterium species V.P.I. 13708. J Lipid Res. 1981 Aug;22(6):891–898. [PubMed] [Google Scholar]
- White B. A., Fricke R. J., Hylemon P. B. 7 beta-Dehydroxylation of ursodeoxycholic acid by whole cells and cell extracts of the intestinal anaerobic bacterium, Eubacterium species V.P.I. 12708. J Lipid Res. 1982 Jan;23(1):145–153. [PubMed] [Google Scholar]
- White B. A., Lipsky R. L., Fricke R. J., Hylemon P. B. Bile acid induction specificity of 7 alpha-dehydroxylase activity in an intestinal Eubacterium species. Steroids. 1980 Jan;35(1):103–109. doi: 10.1016/0039-128x(80)90115-4. [DOI] [PubMed] [Google Scholar]
- White B. A., Paone D. A., Cacciapuoti A. F., Fricke R. J., Mosbach E. H., Hylemon P. B. Regulation of bile acid 7-dehydroxylase activity by NAD+ and NADH in cell extracts of Eubacterium species V.P.I. 12708. J Lipid Res. 1983 Jan;24(1):20–27. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]