Abstract
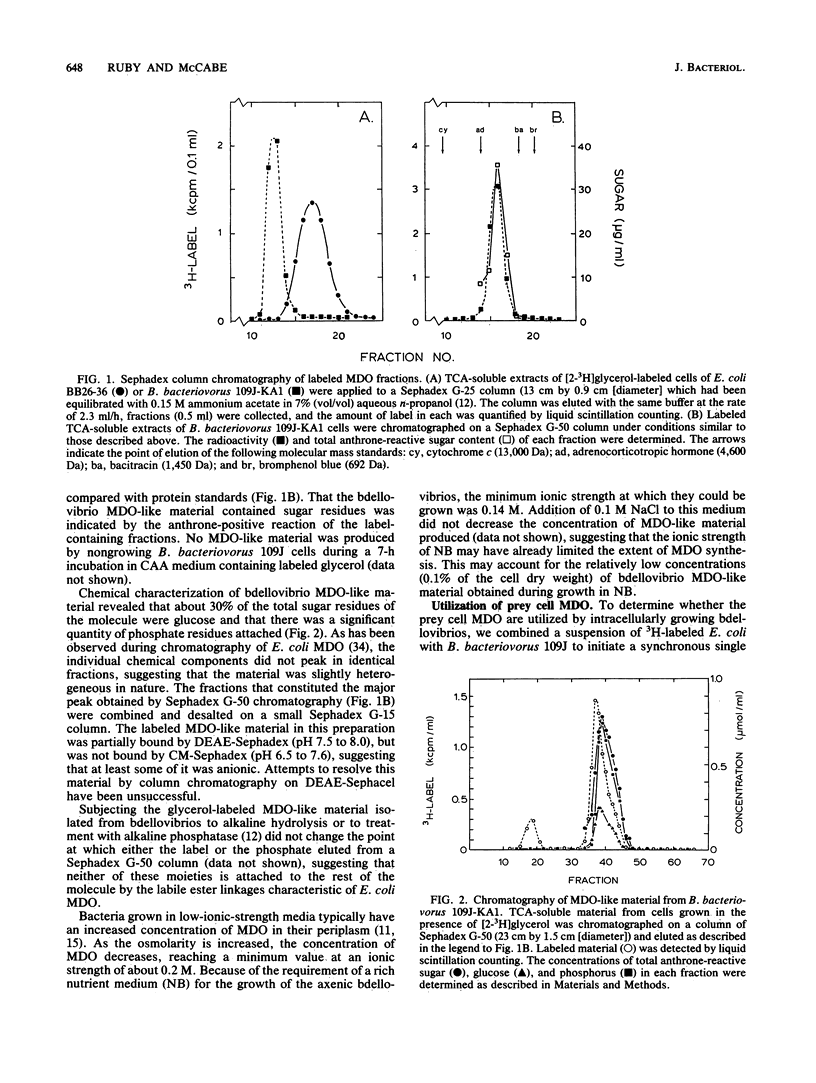
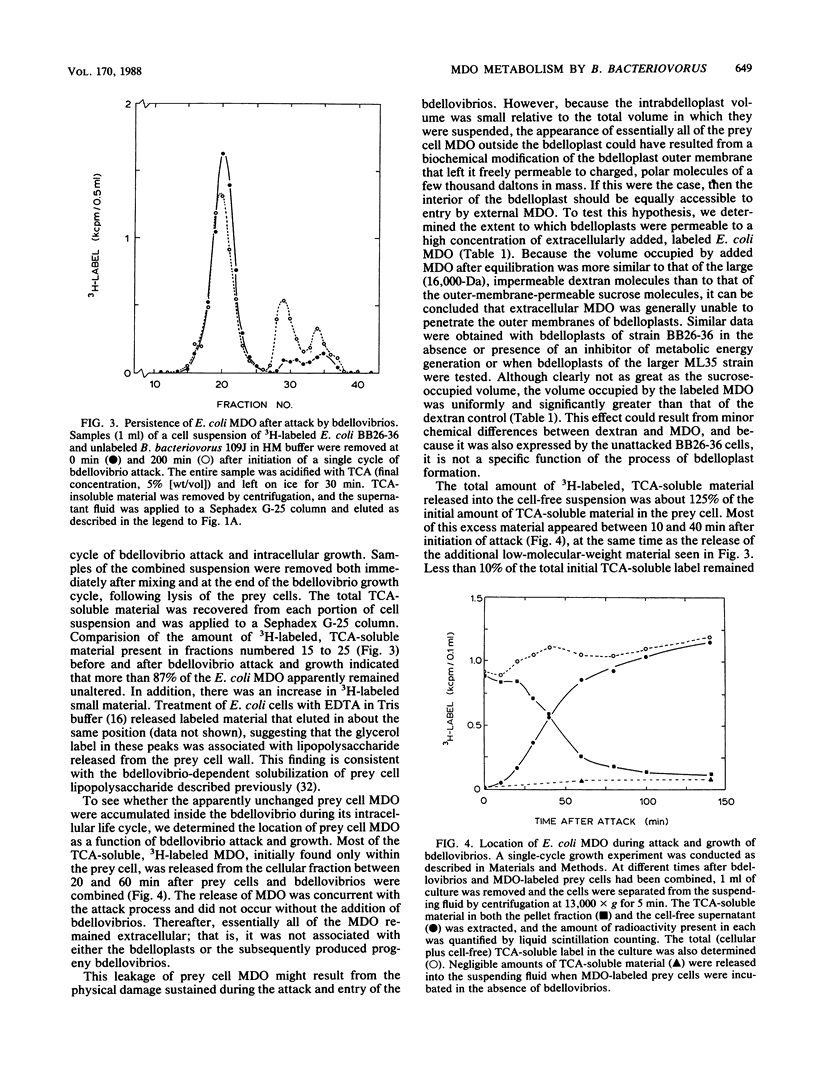
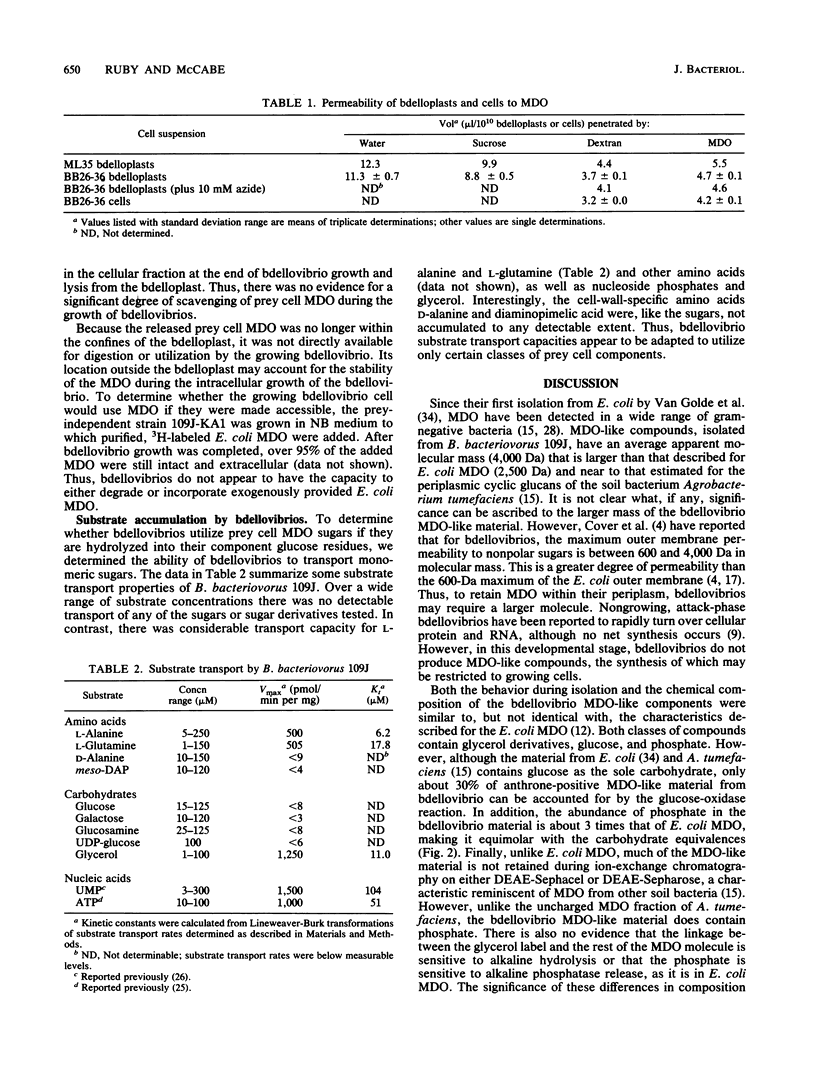
Membrane-derived oligosaccharides (MDO), a class of osmotically active carbohydrates, are the major organic solutes present in the periplasm of Escherichia coli and many other gram-negative bacteria when cells are grown in a medium of low osmolarity. Analyses of growing cells of Bdellovibrio bacteriovorus, a gram-negative predator of other bacteria, have confirmed that they also synthesize a characteristic MDO-like class of oligosaccharides. The natural growth environment of bdellovibrios is the periplasm of other gram-negative bacteria. Because of this location, prey cell MDO constitute a potential source of organic nutrients for growing bdellovibrios. Using cells of E. coli whose MDO were 3H labeled, we examined the extent to which B. bacteriovorus 109J metabolizes these prey cell components. Interestingly, there was neither significant degradation nor incorporation of prey cell MDO by bdellovibrios during the course of their intracellular growth. In fact, bdellovibrios had little capability either to degrade extracellular MDO that was made available to them or to transport glucose, the major monomeric constituent of prey cell MDO. Instead, periplasmic MDO were irreversibly lost to the extracellular environment during the period of bdellovibrio attack and penetration. Thus, although prey cell periplasmic proteins are retained, other important periplasmic components are released early in the bdellovibrio growth cycle. The loss of these MDO may aid in the destabilization of the prey cell plasma membrane, increasing the availability of cytoplasmic constituents to the periplasmic bdellovibrio.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Amemura A., Cabrera-Crespo J. Extracellular oligosaccharides and low-Mr polysaccharides containing (1----2)-beta-D-glucosidic linkages from strains of Xanthomonas, Escherichia coli and Klebsiella pneumoniae. J Gen Microbiol. 1986 Sep;132(9):2443–2452. doi: 10.1099/00221287-132-9-2443. [DOI] [PubMed] [Google Scholar]
- Burnham J. C., Hashimoto T., Conti S. F. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J Bacteriol. 1968 Oct;96(4):1366–1381. doi: 10.1128/jb.96.4.1366-1381.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover W. H., Martinez R. J., Rittenberg S. C. Permeability of the boundary layers of Bdellovibrio bacteriovorus 109J and its bdelloplasts to small hydrophilic molecules. J Bacteriol. 1984 Feb;157(2):385–390. doi: 10.1128/jb.157.2.385-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelking H. M., Seidler R. J. The involvement of extracellular enzymes in the metabolism of Bdellovibrio. Arch Mikrobiol. 1974 Feb 13;95(4):293–304. doi: 10.1007/BF02451770. [DOI] [PubMed] [Google Scholar]
- Gloor L., Klubek B., Seidler R. J. Molecular heterogeneity of the bdellovibrios: metallo and serine proteases unique to each species. Arch Mikrobiol. 1974 Mar 1;95(1):45–56. doi: 10.1007/BF02451747. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Odelson D. A. Metabolism of RNA-ribose by Bdellovibrio bacteriovorus during intraperiplasmic growth on Escherichia coli. J Bacteriol. 1978 Dec;136(3):936–946. doi: 10.1128/jb.136.3.936-946.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Rittenberg S. C. Incorporation of substrate cell lipid A components into the lipopolysaccharide of intraperiplasmically grown Bdellovibrio bacteriovorus. J Bacteriol. 1981 Sep;147(3):860–868. doi: 10.1128/jb.147.3.860-868.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odelson D. A., Patterson M. A., Hespell R. B. Periplasmic enzymes in Bdellovibrio bacteriovorus and Bdellovibrio stolpii. J Bacteriol. 1982 Aug;151(2):756–763. doi: 10.1128/jb.151.2.756-763.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Hespell R. B. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1158–1165. doi: 10.1128/jb.121.3.1158-1165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C. Nonidentity of Bdellovibrio bacteriovorus strains 109D and 109J. J Bacteriol. 1972 Jan;109(1):432–433. doi: 10.1128/jb.109.1.432-433.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson R. A., Rittenberg S. C. Regulated breakdown of Escherichia coli deoxyribonucleic acid during intraperiplasmic growth of Bdellovibrio bacteriovorus 109J. J Bacteriol. 1979 Nov;140(2):620–633. doi: 10.1128/jb.140.2.620-633.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., McCabe J. B. An ATP transport system in the intracellular bacterium, Bdellovibrio bacteriovorus 109J. J Bacteriol. 1986 Sep;167(3):1066–1070. doi: 10.1128/jb.167.3.1066-1070.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., McCabe J. B., Barke J. I. Uptake of intact nucleoside monophosphates by Bdellovibrio bacteriovorus 109J. J Bacteriol. 1985 Sep;163(3):1087–1094. doi: 10.1128/jb.163.3.1087-1094.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Rittenberg S. C. Differentiation after premature release of intraperiplasmically growing Bdellovibrio bacteriovorous. J Bacteriol. 1983 Apr;154(1):32–40. doi: 10.1128/jb.154.1.32-40.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Localization of membrane-derived oligosaccharides in the outer envelope of Escherichia coli and their occurrence in other Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):686–688. doi: 10.1128/jb.137.1.686-688.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969 Nov;100(2):769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Talley B. G., McDade R. L., Jr, Diedrich D. L. Verification of the protein in the outer membrane of Bdellovibrio bacteriovorus as the OmpF protein of its Escherichia coli prey. J Bacteriol. 1987 Feb;169(2):694–698. doi: 10.1128/jb.169.2.694-698.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: N-deacetylation of Escherichia coli peptidoglycan amino sugars. J Bacteriol. 1978 Sep;135(3):1008–1014. doi: 10.1128/jb.135.3.1008-1014.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):998–1007. doi: 10.1128/jb.135.3.998-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Golde L. M. Metabolism of membrane phospholipids and its relation to a novel class of oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1368–1372. doi: 10.1073/pnas.70.5.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]


