Abstract
Bis(5'-adenosyl) tetraphosphate (Ap4A) phosphorylase II (P. Plateau, M. Fromant, J. M. Schmitter, J. M. Buhler, and S. Blanquet, J. Bacteriol. 171:6437-6445, 1989) was obtained in a homogeneous form through a 40,000-fold purification, starting from a Saccharomyces cerevisiae strain devoid of Ap4A phosphorylase I activity. The former enzyme behaves as a 36.8K monomer. As with Ap4A phosphorylase I, the addition of divalent cations is required for the expression of activity. Mn2+, Mg2+, and Ca2+ sustain phosphorolysis by the two enzymes, whereas Co2+ and Cd2+ stimulate only phosphorylase II activity. All bis(5'-nucleosidyl) tetraphosphates assayed (Ap4A, Ap4C, Ap4G, Ap4U, Gp4G, and Gp4U) are substrates of the two enzymes. However, Ap4A phosphorylase II shows a marked preference for A-containing substrates. The two enzymes catalyze adenosine 5'-phosphosulfate phosphorolysis or an exchange reaction between Pi and the beta-phosphate of any nucleoside diphosphate. They can also produce Ap4A at the expense of ATP and ADP. The gene (APA2) encoding Ap4A phosphorylase II was isolated and sequenced. The deduced amino acid sequence shares 60% identity with that of Ap4A phosphorylase I. Disruption of APA2 and/or APA1 shows that none of these genes is essential for the viability of Saccharomyces cerevisiae. The concentrations of all bis(5'-nucleosidyl) tetraphosphates are increased in an apa1 apa2 double mutant, as compared with the parental wild-type strain. The factor of increase is 5 to 50 times, depending on the nucleotide. This observation supports the conclusion that, in vivo, Ap4A phosphorylase II, like Ap4A phosphorylase I, participates in the catabolism rather than the synthesis of the bis(5'-nucleosidyl) tetraphosphates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Nicholas D. J. Adenosine 5'-pyrophosphate sulphurylase in baker's yeast. Biochem J. 1972 Jul;128(3):647–654. doi: 10.1042/bj1280647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. C., Jacobson M. K. Alteration of adenyl dinucleotide metabolism by environmental stress. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2350–2352. doi: 10.1073/pnas.83.8.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. C., Jacobson M. K. Determination of diadenosine 5',5''',-P1,P4-tetraphosphate levels in cultured mammalian cells. Anal Biochem. 1984 Sep;141(2):451–460. doi: 10.1016/0003-2697(84)90070-8. [DOI] [PubMed] [Google Scholar]
- Beauvallet C., Hountondji C., Schmitter J. M. Analytical strategy for determination of active site sequences in aminoacyl-tRNA synthetases. J Chromatogr. 1988 Apr 22;438(2):347–357. doi: 10.1016/s0021-9673(00)90266-8. [DOI] [PubMed] [Google Scholar]
- Blanquet S., Plateau P., Brevet A. The role of zinc in 5',5'-diadenosine tetraphosphate production by aminoacyl-transfer RNA synthetases. Mol Cell Biochem. 1983;52(1):3–11. doi: 10.1007/BF00230583. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Brevet A., Chen J., Lévêque F., Plateau P., Blanquet S. In vivo synthesis of adenylylated bis(5'-nucleosidyl) tetraphosphates (Ap4N) by Escherichia coli aminoacyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8275–8279. doi: 10.1073/pnas.86.21.8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevet A., Coste H., Fromant M., Plateau P., Blanquet S. Yeast diadenosine 5',5'''-P1,P4-tetraphosphate alpha,beta-phosphorylase behaves as a dinucleoside tetraphosphate synthetase. Biochemistry. 1987 Jul 28;26(15):4763–4768. doi: 10.1021/bi00389a025. [DOI] [PubMed] [Google Scholar]
- Brevet A., Plateau P., Best-Belpomme M., Blanquet S. Variation of Ap4A and other dinucleoside polyphosphates in stressed Drosophila cells. J Biol Chem. 1985 Dec 15;260(29):15566–15570. [PubMed] [Google Scholar]
- Coste H., Brevet A., Plateau P., Blanquet S. Non-adenylylated bis(5'-nucleosidyl) tetraphosphates occur in Saccharomyces cerevisiae and in Escherichia coli and accumulate upon temperature shift or exposure to cadmium. J Biol Chem. 1987 Sep 5;262(25):12096–12103. [PubMed] [Google Scholar]
- Garrison P. N., Mathis S. A., Barnes L. D. Changes in diadenosine tetraphosphate levels in Physarum polycephalum with different oxygen concentrations. J Bacteriol. 1989 Mar;171(3):1506–1512. doi: 10.1128/jb.171.3.1506-1512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A., Blanquet S. Diadenosine 5',5'''-P1, P4-tetraphosphate alpha, beta-phosphorylase from yeast supports nucleoside diphosphate-phosphate exchange. J Biol Chem. 1986 May 5;261(13):5943–5946. [PubMed] [Google Scholar]
- Guranowski A., Blanquet S. Phosphorolytic cleavage of diadenosine 5',5'''-P1,P4-tetraphosphate. Properties of homogeneous diadenosine 5',5'''-P1,P4-tetraphosphate alpha, beta-phosphorylase from Saccharomyces cerevisiae. J Biol Chem. 1985 Mar 25;260(6):3542–3547. [PubMed] [Google Scholar]
- Guranowski A., Just G., Holler E., Jakubowski H. Synthesis of diadenosine 5',5'''-P1,P4-tetraphosphate (AppppA) from adenosine 5'-phosphosulfate and adenosine 5'-triphosphate catalyzed by yeast AppppA phosphorylase. Biochemistry. 1988 Apr 19;27(8):2959–2964. doi: 10.1021/bi00408a044. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveque F., Blanchin-Roland S., Fayat G., Plateau P., Blanquet S. Design and characterization of Escherichia coli mutants devoid of Ap4N-hydrolase activity. J Mol Biol. 1990 Mar 20;212(2):319–329. doi: 10.1016/0022-2836(90)90127-8. [DOI] [PubMed] [Google Scholar]
- Mann C., Buhler J. M., Treich I., Sentenac A. RPC40, a unique gene for a subunit shared between yeast RNA polymerases A and C. Cell. 1987 Feb 27;48(4):627–637. doi: 10.1016/0092-8674(87)90241-8. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie A. Determination of diadenosine tetraphosphate (Ap4A) levels in subpicomole quantities by a phosphodiesterase luciferin--luciferase coupled assay: application as a specific assay for diadenosine tetraphosphatase. Anal Biochem. 1981 Aug;115(2):302–307. doi: 10.1016/0003-2697(81)90009-9. [DOI] [PubMed] [Google Scholar]
- Ogilvie A., Jakob P. Diadenosine 5',5"'-P1,P3-triphosphate in eukaryotic cells: identification and quantitation. Anal Biochem. 1983 Oct 15;134(2):382–392. doi: 10.1016/0003-2697(83)90313-5. [DOI] [PubMed] [Google Scholar]
- Parent S. A., Fenimore C. M., Bostian K. A. Vector systems for the expression, analysis and cloning of DNA sequences in S. cerevisiae. Yeast. 1985 Dec;1(2):83–138. doi: 10.1002/yea.320010202. [DOI] [PubMed] [Google Scholar]
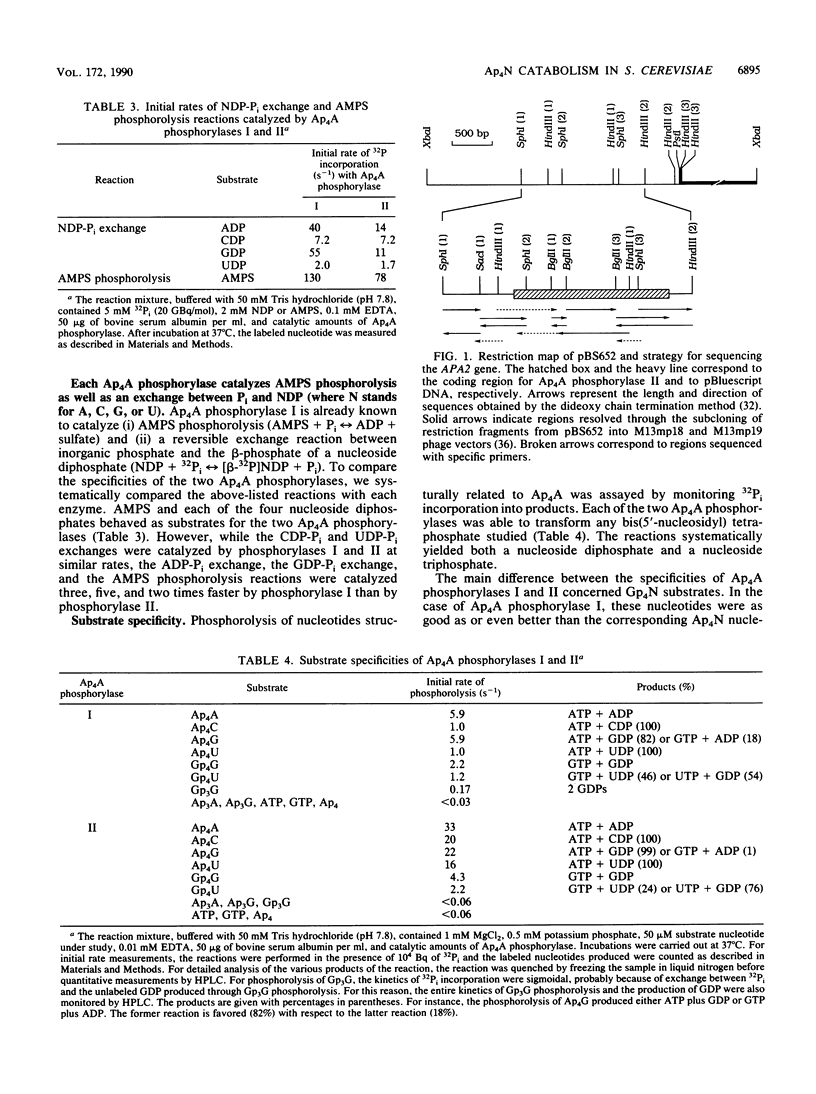
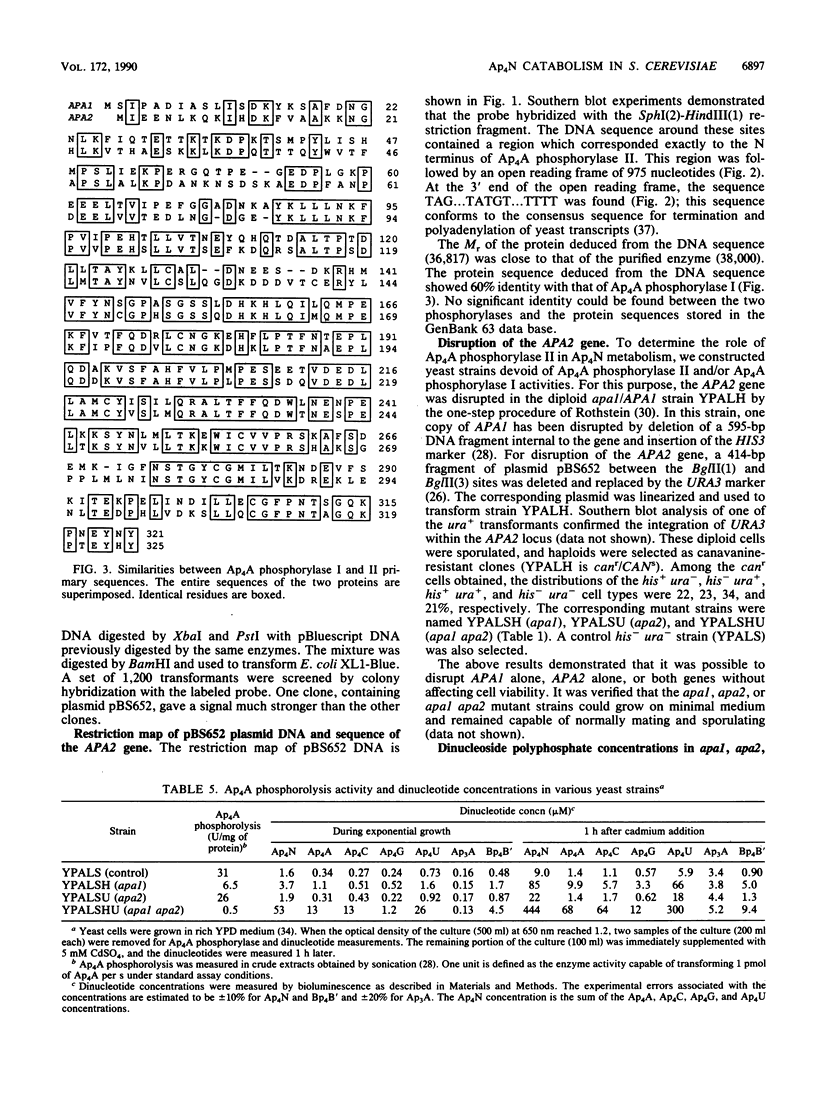
- Plateau P., Fromant M., Schmitter J. M., Buhler J. M., Blanquet S. Isolation, characterization, and inactivation of the APA1 gene encoding yeast diadenosine 5',5'''-P1,P4-tetraphosphate phosphorylase. J Bacteriol. 1989 Dec;171(12):6437–6445. doi: 10.1128/jb.171.12.6437-6445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Separation of the two enzymatic phases in active sulfate synthesis. J Biol Chem. 1958 Sep;233(3):681–685. [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Warner A. H., Finamore F. J. Isolation, purification, and characterization of P1,P3-diguanosine 5'-triphosphate from brine shrimp eggs. Biochim Biophys Acta. 1965 Dec 9;108(4):525–530. doi: 10.1016/0005-2787(65)90049-3. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]


