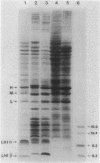
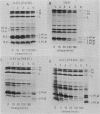
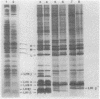
Abstract
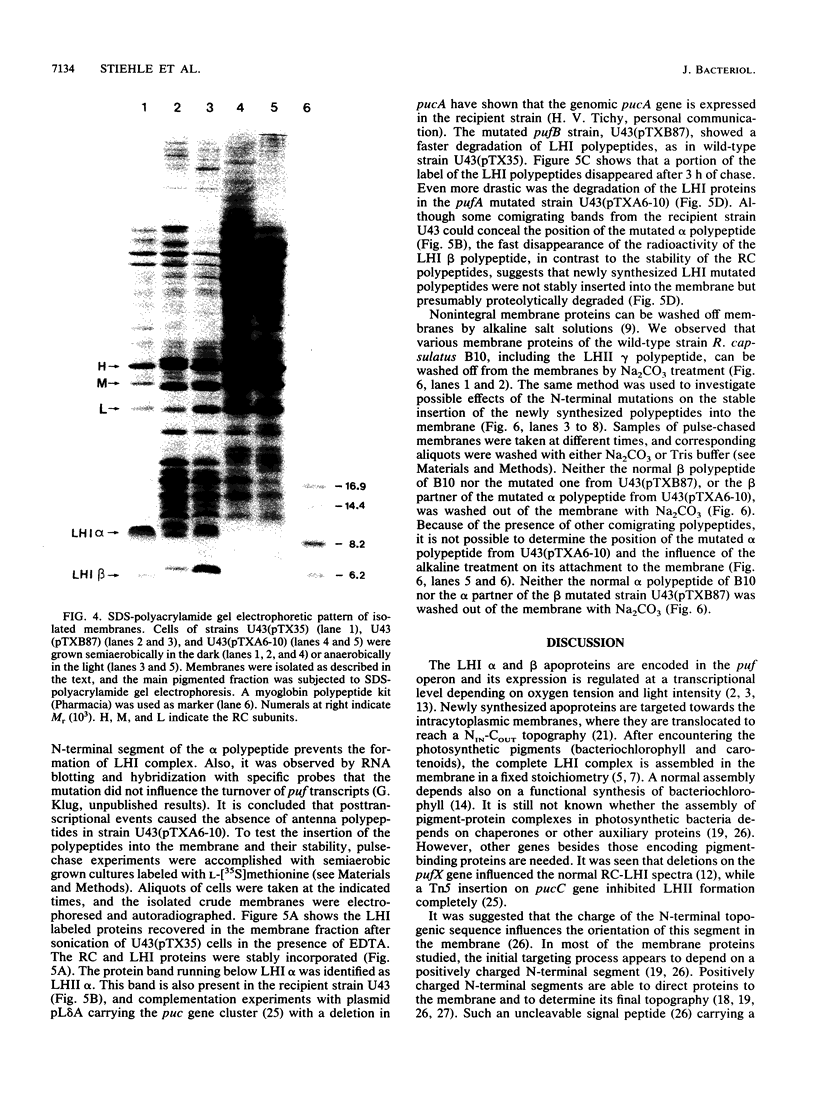
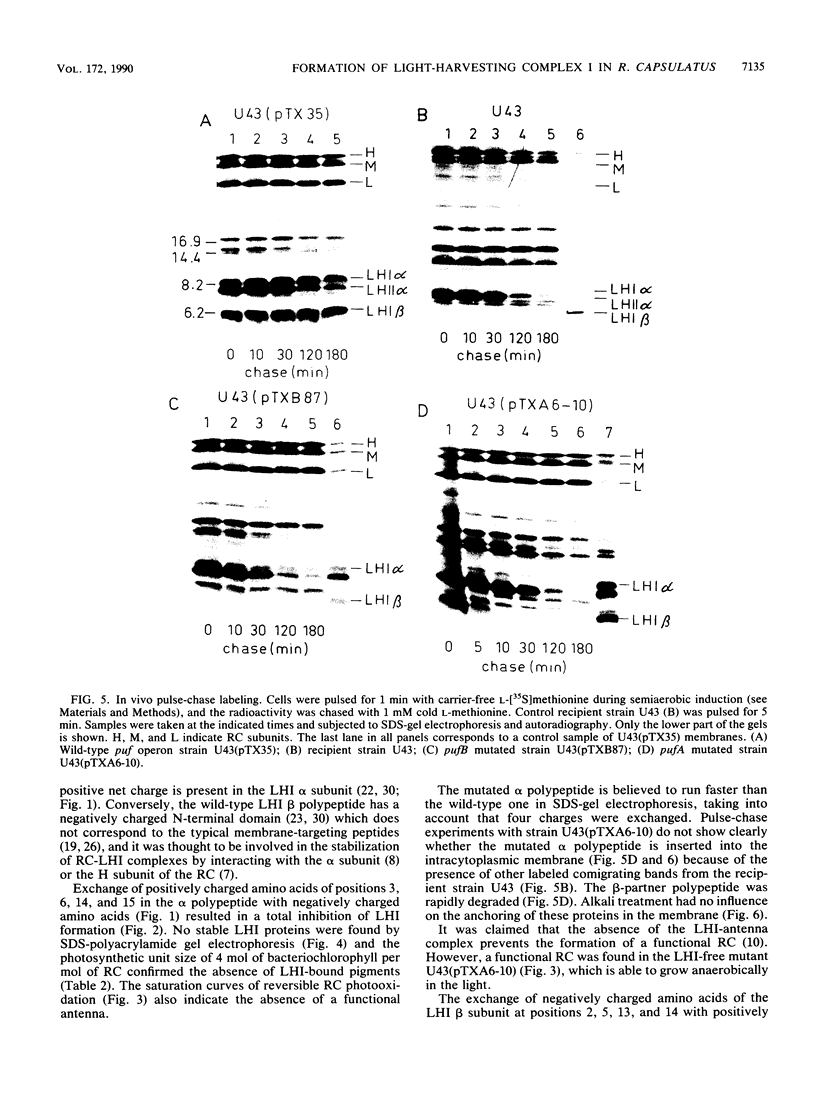
Light-harvesting complex I (LHI) of Rhodobacter capsulatus contains bacteriochlorophyll and carotenoids which are noncovalently bound to two different apoproteins (alpha and beta polypeptides) carrying oppositely charged N-terminal ends. The contribution of these charged segments to the assembly of LHI was studied with mutants having oppositely charged amino acids in the alpha or beta polypeptide. The influence of these mutations on the insertion and assembly process of the LHI complex was investigated by means of spectroscopic analysis of isolated intracytoplasmic membranes and pulse-chase experiments. Exchange of four positively charged amino acids to negatively charged amino acids on the N-terminal domain of the alpha subunit inhibited completely the assembly of the LHI complex. Although this mutant has no antenna, the reaction center is active and the cells were able to grow anaerobically in the light. Conversely, mutation of the four negatively charged amino acids of the N-terminal segment of the beta polypeptide did not prevent the assembly of the LHI complex, although the stability of the complex and the size of the photosynthetic unit were affected. The presence of the mutated beta polypeptide was confirmed by protein sequencing.
Full text
PDF

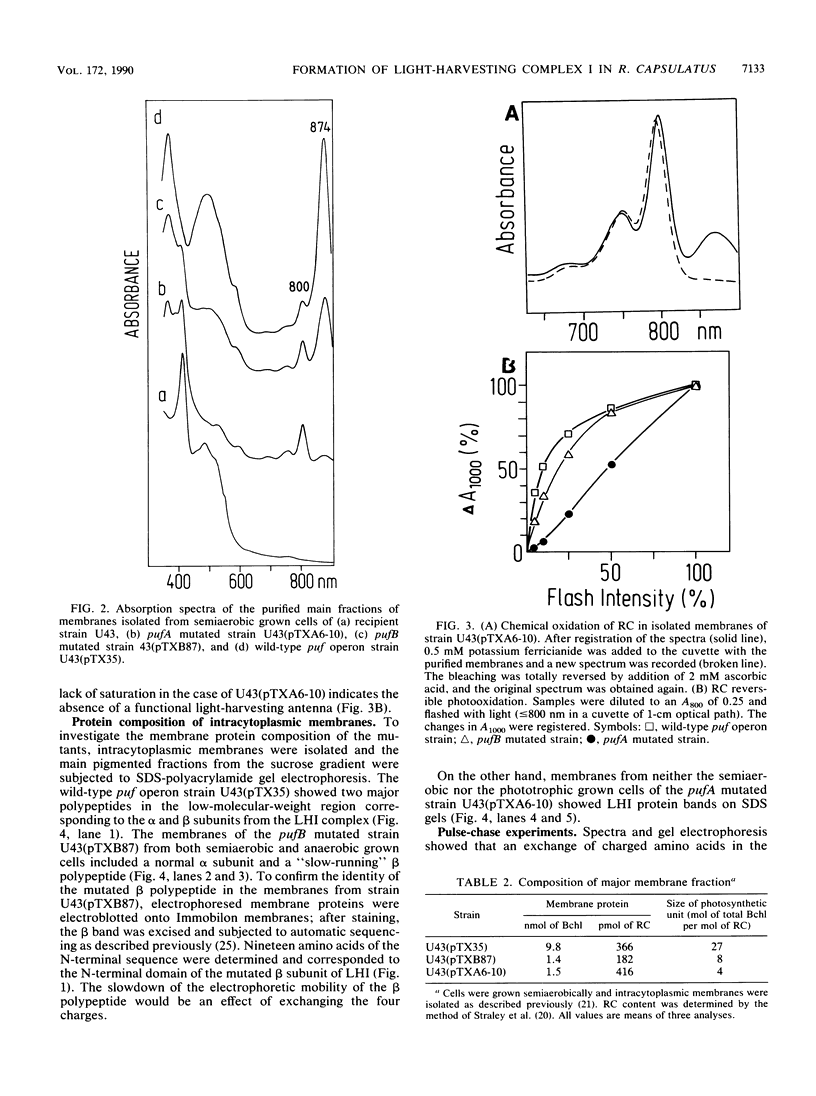


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer C. E., Marrs B. L. Rhodobacter capsulatus puf operon encodes a regulatory protein (PufQ) for bacteriochlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7074–7078. doi: 10.1073/pnas.85.19.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Clark W. G., Davidson E., Marrs B. L. Variation of levels of mRNA coding for antenna and reaction center polypeptides in Rhodopseudomonas capsulata in response to changes in oxygen concentration. J Bacteriol. 1984 Mar;157(3):945–948. doi: 10.1128/jb.157.3.945-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews G. Structure and functional organization of light-harvesting complexes and photochemical reaction centers in membranes of phototrophic bacteria. Microbiol Rev. 1985 Mar;49(1):59–70. doi: 10.1128/mr.49.1.59-70.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörge B., Klug G., Gad'on N., Cohen S. N., Drews G. Effects on the formation of antenna complex B870 of Rhodobacter capsulatus by exchange of charged amino acids in the N-terminal domain of the alpha and beta pigment-binding proteins. Biochemistry. 1990 Aug 21;29(33):7754–7758. doi: 10.1021/bi00485a026. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Pleiotropic effects of localized Rhodobacter capsulatus puf operon deletions on production of light-absorbing pigment-protein complexes. J Bacteriol. 1988 Dec;170(12):5814–5821. doi: 10.1128/jb.170.12.5814-5821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Kaufmann N., Drews G. Gene expression of pigment-binding proteins of the bacterial photosynthetic apparatus: Transcription and assembly in the membrane of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6485–6489. doi: 10.1073/pnas.82.19.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nieth K. F., Drews G. Formation of reaction centers and light-harvesting bacteriochlorophyll-protein complexes in Rhodopseudomonas capsulata. Arch Microbiol. 1975 Jun 20;104(1):77–82. doi: 10.1007/BF00447303. [DOI] [PubMed] [Google Scholar]
- Puziss J. W., Fikes J. D., Bassford P. J., Jr Analysis of mutational alterations in the hydrophilic segment of the maltose-binding protein signal peptide. J Bacteriol. 1989 May;171(5):2303–2311. doi: 10.1128/jb.171.5.2303-2311.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Tadros M. H., Suter F., Seydewitz H. H., Witt I., Zuber H., Drews G. Isolation and complete amino-acid sequence of the small polypeptide from light-harvesting pigment-protein complex I (B870) of Rhodopseudomonas capsulata. Eur J Biochem. 1984 Jan 2;138(1):209–212. doi: 10.1111/j.1432-1033.1984.tb07902.x. [DOI] [PubMed] [Google Scholar]
- Tichy H. V., Oberlé B., Stiehle H., Schiltz E., Drews G. Genes downstream from pucB and pucA are essential for formation of the B800-850 complex of Rhodobacter capsulatus. J Bacteriol. 1989 Sep;171(9):4914–4922. doi: 10.1128/jb.171.9.4914-4922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Mizushima S. Introduction of basic amino acid residues after the signal peptide inhibits protein translocation across the cytoplasmic membrane of Escherichia coli. Relation to the orientation of membrane proteins. J Biol Chem. 1988 Dec 25;263(36):19690–19696. [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S., Bylina E. J. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38(1-3):19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]