Abstract
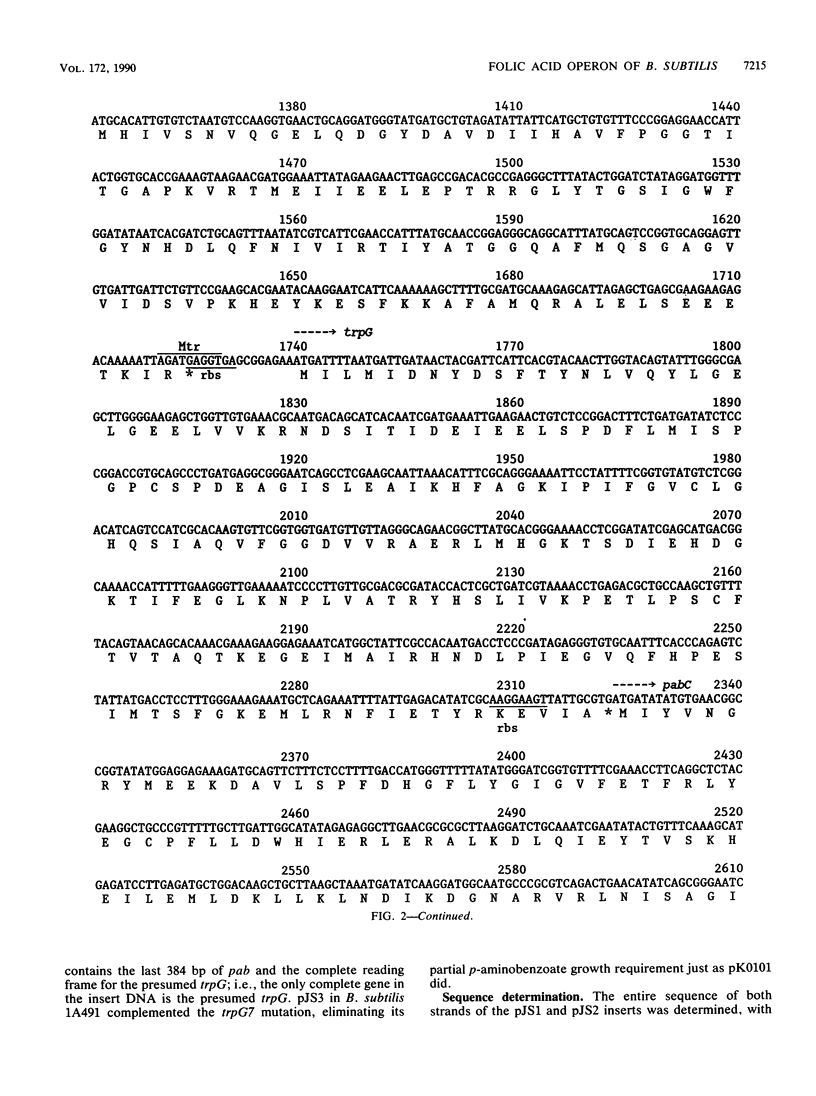
McDonald and Burke (J. Bacteriol. 149:391-394, 1982) previously cloned a sulfanilamide-resistance gene, sul, residing on a 4.9-kb segment of Bacillus subtilis chromosomal DNA, into plasmid pUB110. In this study we determined the nucleotide sequence of the entire 4.9-kb fragment. Genes identified on the fragment include pab, trpG, pabC, sul, one complete unidentified open reading frame, and one incomplete unidentified open reading frame. The first three of these genes, pab, trpG, and pabC, are required for synthesis of p-aminobenzoic acid. The trpG gene encodes an amphibolic glutamine amidotransferase required for synthesis of both p-aminobenzoate and anthranilate, the latter an intermediate in the tryptophan biosynthetic pathway. The pabC gene may encode a B. subtilis analog of enzyme X, an enzyme needed for p-aminobenzoate synthesis in Escherichia coli. The sul gene probably encodes dihydropteroate synthase, the enzyme responsible for formation of 7,8-dihydropteroate, the immediate precursor of folic acid. All six of the cloned genes are arranged in a single operon. Since all four of the identified genes are needed for folate biosynthesis, we refer to this operon as a folic acid operon. Expression of the trpG gene is known to be negatively controlled by tryptophan. We propose that this regulation is at the level of translation. This hypothesis is supported by the finding of an apparent Mtr-binding site which overlaps with the trpG ribosome-binding site.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Crawford I. P. Le groupe des gènes régissant la biosynthèse du tryptophane chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):93–96. [PubMed] [Google Scholar]
- Bakhiet N., Stahly D. P. Studies on transfection and transformation of protoplasts of Bacillus larvae, Bacillus subtilis, and Bacillus popilliae. Appl Environ Microbiol. 1985 Mar;49(3):577–581. doi: 10.1128/aem.49.3.577-581.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Metabolic and nutritional factors influencing the development of competence for transfection of Bacillus subtilis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):370–378. [PMC free article] [PubMed] [Google Scholar]
- Buvinger W. E., Stone L. C., Heath H. E. Biochemical genetics of tryptophan synthesis in Pseudomonas acidovorans. J Bacteriol. 1981 Jul;147(1):62–68. doi: 10.1128/jb.147.1.62-68.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C., Whitt D. D. The isolation and genetic characterization of mutants of the tryptophan system of Bacillus subtilis. Genetics. 1969 Jul;62(3):445–460. doi: 10.1093/genetics/62.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3240–3244. doi: 10.1073/pnas.70.11.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- Essar D. W., Eberly L., Han C. Y., Crawford I. P. DNA sequences and characterization of four early genes of the tryptophan pathway in Pseudomonas aeruginosa. J Bacteriol. 1990 Feb;172(2):853–866. doi: 10.1128/jb.172.2.853-866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Dubnau D. Construction and properties of chimeric plasmids in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1428–1432. doi: 10.1073/pnas.75.3.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D. J., Band L., Shimotsu H. Nucleotide sequence of the Bacillus subtilis tryptophan operon. Gene. 1985;34(2-3):169–177. doi: 10.1016/0378-1119(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Roth C. W., Crawford I. P., Nester E. W. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):38–45. doi: 10.1128/jb.105.1.38-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanescu S., Surdeanu M., Della Latta P., Novick R. Incompatibility and molecular relationships between small Staphylococcal plasmids carrying the same resistance marker. Plasmid. 1978 Sep;1(4):468–479. doi: 10.1016/0147-619x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Kane J. F., Holmes W. M., Jensen R. A. Metabolic interlock. The dual function of a folate pathway gene as an extra-operonic gene of tryptophan biosynthesis. J Biol Chem. 1972 Mar 10;247(5):1587–1596. [PubMed] [Google Scholar]
- Kane J. F. Regulation of a common amidotransferase subunit. J Bacteriol. 1977 Nov;132(2):419–425. doi: 10.1128/jb.132.2.419-425.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. B., Goncharoff P., Seibold A. M., Nichols B. P. Nucleotide sequence of the Acinetobacter calcoaceticus trpGDC gene cluster. Mol Biol Evol. 1984 Nov;1(6):456–472. doi: 10.1093/oxfordjournals.molbev.a040331. [DOI] [PubMed] [Google Scholar]
- Kaplan J. B., Merkel W. K., Nichols B. P. Evolution of glutamine amidotransferase genes. Nucleotide sequences of the pabA genes from Salmonella typhimurium, Klebsiella aerogenes and Serratia marcescens. J Mol Biol. 1985 Jun 5;183(3):327–340. doi: 10.1016/0022-2836(85)90004-x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu S., Ogawa T., Ogawa H., Kagamiyama H. Branched-chain amino acid aminotransferase of Escherichia coli: nucleotide sequence of the ilvE gene and the deduced amino acid sequence. J Biochem. 1985 Apr;97(4):993–999. doi: 10.1093/oxfordjournals.jbchem.a135176. [DOI] [PubMed] [Google Scholar]
- Kuroda M. I., Henner D., Yanofsky C. cis-acting sites in the transcript of the Bacillus subtilis trp operon regulate expression of the operon. J Bacteriol. 1988 Jul;170(7):3080–3088. doi: 10.1128/jb.170.7.3080-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M. I., Shimotsu H., Henner D. J., Yanofsky C. Regulatory elements common to the Bacillus pumilus and Bacillus subtilis trp operons. J Bacteriol. 1986 Sep;167(3):792–798. doi: 10.1128/jb.167.3.792-798.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini L. M., Brentano S. T., Donelson J. E. A DNA sequence analysis package for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):605–614. doi: 10.1093/nar/12.1part2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez P., Espinosa M., Greenberg B., Lacks S. A. Sulfonamide resistance in Streptococcus pneumoniae: DNA sequence of the gene encoding dihydropteroate synthase and characterization of the enzyme. J Bacteriol. 1987 Sep;169(9):4320–4326. doi: 10.1128/jb.169.9.4320-4326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McDonald K. O., Burke W. F., Jr Cloning of the Bacillus subtilis sulfanilamide resistance gene in Bacillus subtilis. J Bacteriol. 1982 Jan;149(1):391–394. doi: 10.1128/jb.149.1.391-394.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. P., Seibold A. M., Doktor S. Z. para-aminobenzoate synthesis from chorismate occurs in two steps. J Biol Chem. 1989 May 25;264(15):8597–8601. [PubMed] [Google Scholar]
- Ortiz P. J., Hotchkiss R. D. The enzymatic synthesis of dihydrofolate and dihydropteroate in cell-free preparations from wild-type and sulfonamide-resistant pneumococcus. Biochemistry. 1966 Jan;5(1):67–74. doi: 10.1021/bi00865a010. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. A physical and functional analysis of Tn917, a Streptococcus transposon in the Tn3 family that functions in Bacillus. Plasmid. 1984 Sep;12(2):119–138. doi: 10.1016/0147-619x(84)90058-1. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Curtis C. A., de Lencastre H. Use of integrational plasmid vectors to demonstrate the polycistronic nature of a transcriptional unit (spoIIA) required for sporulation of Bacillus subtilis. J Gen Microbiol. 1984 Aug;130(8):2123–2136. doi: 10.1099/00221287-130-8-2123. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey D. P., Brown G. M. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J Biol Chem. 1969 Mar 25;244(6):1582–1592. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Mapping of the tryptophan genes of Acinetobacter calcoaceticus by transformation. J Bacteriol. 1972 Nov;112(2):797–805. doi: 10.1128/jb.112.2.797-805.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotsu H., Kuroda M. I., Yanofsky C., Henner D. J. Novel form of transcription attenuation regulates expression the Bacillus subtilis tryptophan operon. J Bacteriol. 1986 May;166(2):461–471. doi: 10.1128/jb.166.2.461-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yousaf S. I., Carroll A. R., Clarke B. E. A new and improved method for 3'-end labelling DNA using [alpha-32P]ddATP. Gene. 1984 Mar;27(3):309–313. doi: 10.1016/0378-1119(84)90075-1. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Ebbole D. J. Organization and regulation of genes encoding biosynthetic enzymes in Bacillus subtilis. J Biol Chem. 1988 Feb 5;263(4):1595–1598. [PubMed] [Google Scholar]


