Abstract
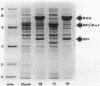
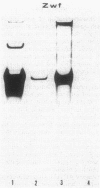
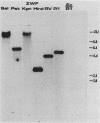
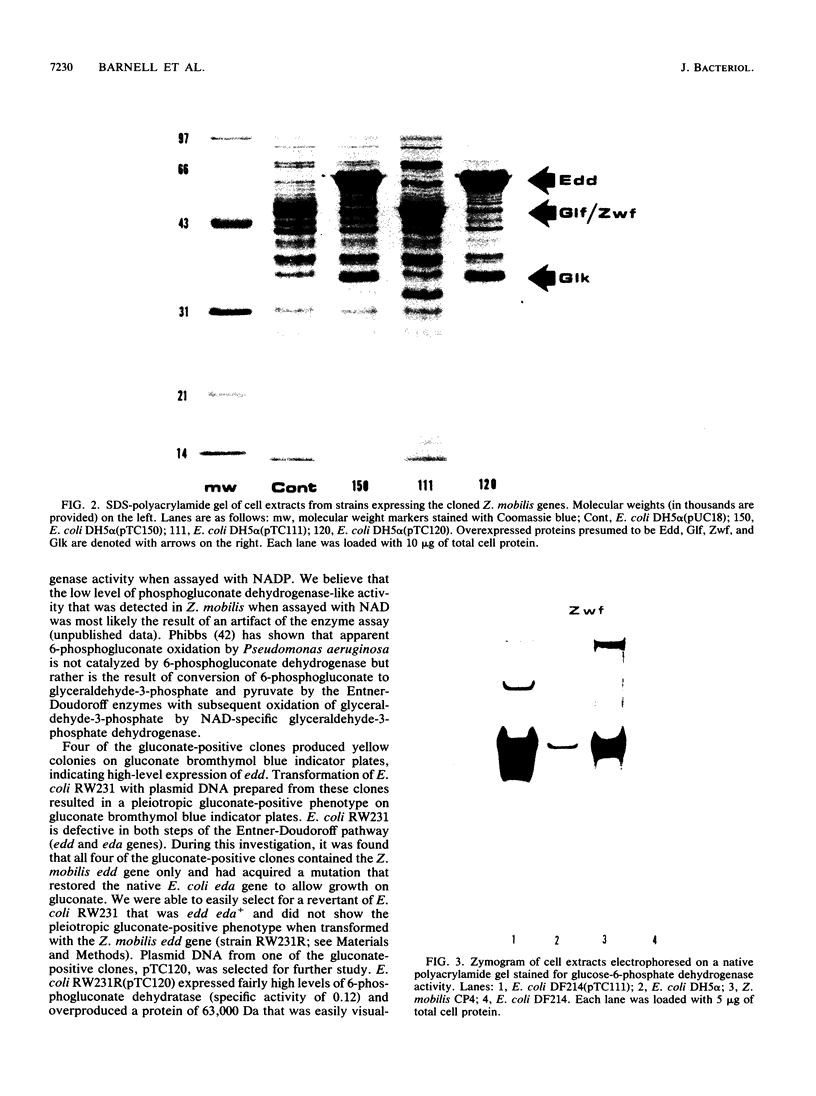
The Zymomonas mobilis genes that encode glucose-6-phosphate dehydrogenase (zwf), 6-phosphogluconate dehydratase (edd), and glucokinase (glk) were cloned independently by genetic complementation of specific defects in Escherichia coli metabolism. The identity of these cloned genes was confirmed by various biochemical means. Nucleotide sequence analysis established that these three genes are clustered on the genome and revealed an additional open reading frame in this region that has significant amino acid identity to the E. coli xylose-proton symporter and the human glucose transporter. On the basis of this evidence and structural analysis of the deduced primary amino acid sequence, this gene is believed to encode the Z. mobilis glucose-facilitated diffusion protein, glf. The four genes in the 6-kb cluster are organized in the order glf, zwf, edd, glk. The glf and zwf genes are separated by 146 bp. The zwf and edd genes overlap by 8 bp, and their expression may be translationally coupled. The edd and glk genes are separated by 203 bp. The glk gene is followed by tandem transcriptional terminators. The four genes appear to be organized in an operon. Such an arrangement of the genes that govern glucose uptake and the first three steps of the Entner-Doudoroff glycolytic pathway provides the organism with a mechanism for carefully regulating the levels of the enzymes that control carbon flux into the pathway.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albig W., Entian K. D. Structure of yeast glucokinase, a strongly diverged specific aldo-hexose-phosphorylating isoenzyme. Gene. 1988 Dec 15;73(1):141–152. doi: 10.1016/0378-1119(88)90320-4. [DOI] [PubMed] [Google Scholar]
- Andreone T. L., Printz R. L., Pilkis S. J., Magnuson M. A., Granner D. K. The amino acid sequence of rat liver glucokinase deduced from cloned cDNA. J Biol Chem. 1989 Jan 5;264(1):363–369. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. V., 2nd, Wolf R. E., Jr Growth rate-dependent regulation of 6-phosphogluconate dehydrogenase level in Escherichia coli K-12: beta-galactosidase expression in gnd-lac operon fusion strains. J Bacteriol. 1983 Feb;153(2):771–781. doi: 10.1128/jb.153.2.771-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow K. D., Collins J. G., Norton R. S., Rogers P. L., Smith G. M. 31P nuclear magnetic resonance studies of the fermentation of glucose to ethanol by Zymomonas mobilis. J Biol Chem. 1984 May 10;259(9):5711–5716. [PubMed] [Google Scholar]
- Belaich J. P., Senez J. C., Murgier M. Microcalorimetric study of glucose permeation in microbial cells. J Bacteriol. 1968 May;95(5):1750–1757. doi: 10.1128/jb.95.5.1750-1757.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadbhade M. M., Adams M. J., Flynn T. G., Levy H. R. Sequence identity between a lysine-containing peptide from Leuconostoc mesenteroides glucose-6-phosphate dehydrogenase and an active site peptide from human erythrocyte glucose-6-phosphate dehydrogenase. FEBS Lett. 1987 Jan 26;211(2):243–246. doi: 10.1016/0014-5793(87)81445-x. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh J. A., Middendorf L. R., Grone D. L., Ruth J. L. Continuous, on-line DNA sequencing using oligodeoxynucleotide primers with multiple fluorophores. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5610–5614. doi: 10.1073/pnas.85.15.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Ingram L. O. Similarity of Escherichia coli propanediol oxidoreductase (fucO product) and an unusual alcohol dehydrogenase from Zymomonas mobilis and Saccharomyces cerevisiae. J Bacteriol. 1989 Jul;171(7):3754–3759. doi: 10.1128/jb.171.7.3754-3759.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Osman Y. A., Konnan J. I., Hoffmann E. M., Ingram L. O. Promoter and nucleotide sequences of the Zymomonas mobilis pyruvate decarboxylase. J Bacteriol. 1987 Mar;169(3):949–954. doi: 10.1128/jb.169.3.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T., Sewell G. W., Osman Y. A., Ingram L. O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987 Jun;169(6):2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. L., Cox B. J., Fidanza V., Calhoun D. H. The complete nucleotide sequence of the ilvGMEDA cluster of Escherichia coli K-12. Gene. 1987;56(2-3):185–198. doi: 10.1016/0378-1119(87)90136-3. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. O., Henderson P. J. The cloning and DNA sequence of the gene xylE for xylose-proton symport in Escherichia coli K12. J Biol Chem. 1987 Oct 15;262(29):13928–13932. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarco A. A., Romano A. H. d-Glucose Transport System of Zymomonas mobilis. Appl Environ Microbiol. 1985 Jan;49(1):151–157. doi: 10.1128/aem.49.1.151-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- Eddy C. K., Mejia J. P., Conway T., Ingram L. O. Differential expression of gap and pgk genes within the gap operon of Zymomonas mobilis. J Bacteriol. 1989 Dec;171(12):6549–6554. doi: 10.1128/jb.171.12.6549-6554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Yamaguchi S., Shimosaka M., Murata K., Kimura A. Cloning of the glucokinase gene in Escherichia coli B. J Bacteriol. 1983 Nov;156(2):922–925. doi: 10.1128/jb.156.2.922-925.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Krieg N. R., Phibbs P. V., Jr Transport and catabolism of D-fructose by Spirillum itersomii. J Bacteriol. 1974 Jan;117(1):144–150. doi: 10.1128/jb.117.1.144-150.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Osman Y. A., Conway T., Bonetti S. J., Ingram L. O. Glycolytic flux in Zymomonas mobilis: enzyme and metabolite levels during batch fermentation. J Bacteriol. 1987 Aug;169(8):3726–3736. doi: 10.1128/jb.169.8.3726-3736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTE D., LUH W., BUECHER T. A constant-proportion group in the enzyme activity pattern of the Embden-Meyerhof chain. Biochem Biophys Res Commun. 1962 Jun 4;7:419–424. doi: 10.1016/0006-291x(62)90327-3. [DOI] [PubMed] [Google Scholar]
- Persico M. G., Viglietto G., Martini G., Toniolo D., Paonessa G., Moscatelli C., Dono R., Vulliamy T., Luzzatto L., D'Urso M. Isolation of human glucose-6-phosphate dehydrogenase (G6PD) cDNA clones: primary structure of the protein and unusual 5' non-coding region. Nucleic Acids Res. 1986 Mar 25;14(6):2511–2522. doi: 10.1093/nar/14.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond J. L., Eddy C. K., Mackenzie K. F., Conway T., Borecky D. J., Ingram L. O. Cloning, sequencing, and characterization of the principal acid phosphatase, the phoC+ product, from Zymomonas mobilis. J Bacteriol. 1989 Feb;171(2):767–774. doi: 10.1128/jb.171.2.767-774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopes R. K., Griffiths-Smith K. Use of differential dye-ligand chromatography with affinity elution for enzyme purification: 6-phosphogluconate dehydratase from Zymomonas mobilis. Anal Biochem. 1984 Feb;136(2):530–534. doi: 10.1016/0003-2697(84)90257-4. [DOI] [PubMed] [Google Scholar]
- Scopes R. K., Testolin V., Stoter A., Griffiths-Smith K., Algar E. M. Simultaneous purification and characterization of glucokinase, fructokinase and glucose-6-phosphate dehydrogenase from Zymomonas mobilis. Biochem J. 1985 Jun 15;228(3):627–634. doi: 10.1042/bj2280627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swings J., De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977 Mar;41(1):1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J., Gerstenberger P. D., Goldberg D. E., Gociar E., Orozco de Silva A., Fraenkel D. G. ColE1 hybrid plasmids for Escherichia coli genes of glycolysis and the hexose monophosphate shunt. J Bacteriol. 1979 Jan;137(1):502–506. doi: 10.1128/jb.137.1.502-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinopal R. T., Hillman J. D., Schulman H., Reznikoff W. S., Fraenkel D. G. New phosphoglucose isomerase mutants of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1172–1174. doi: 10.1128/jb.122.3.1172-1174.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. E., Jr Integration of specialized transducing bacteriophage lambda cI857 St68 h80 dgnd his by an unusual pathway promotes formation of deletions and generates a new translocatable element. J Bacteriol. 1980 May;142(2):588–602. doi: 10.1128/jb.142.2.588-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Prather D. M., Shea F. M. Growth-rate-dependent alteration of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase levels in Escherichia coli K-12. J Bacteriol. 1979 Sep;139(3):1093–1096. doi: 10.1128/jb.139.3.1093-1096.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]