Abstract
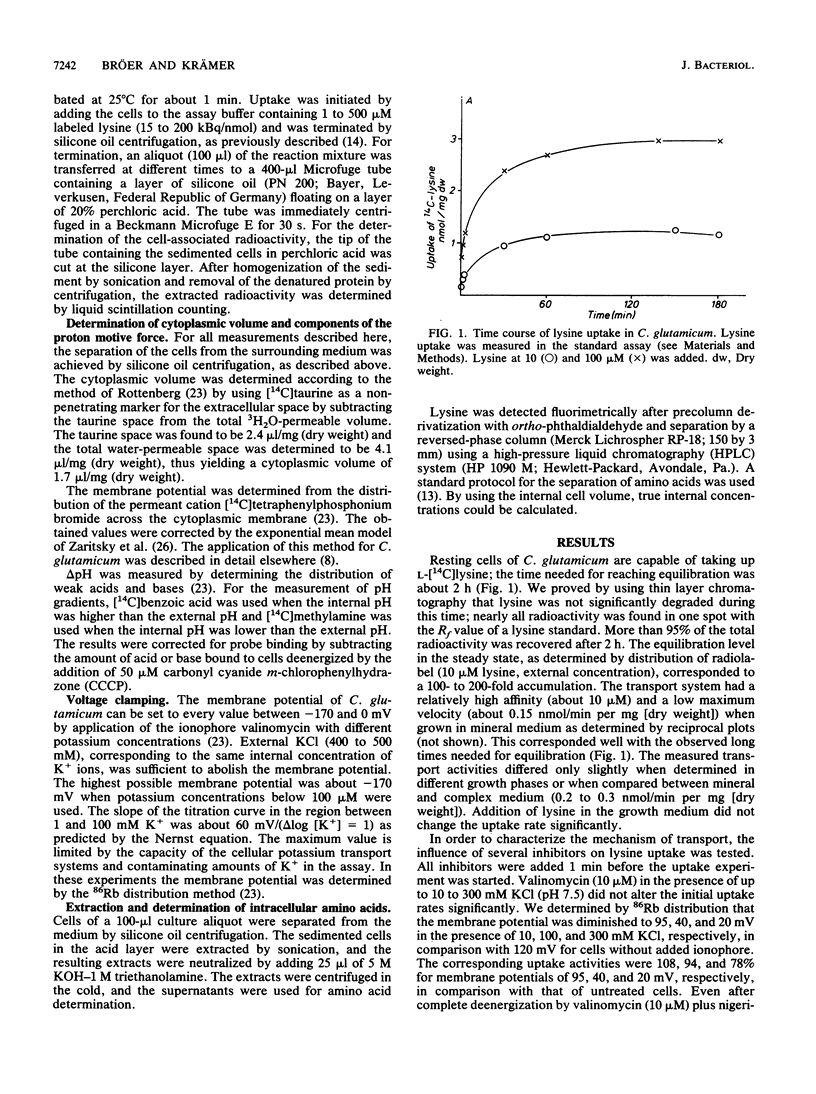
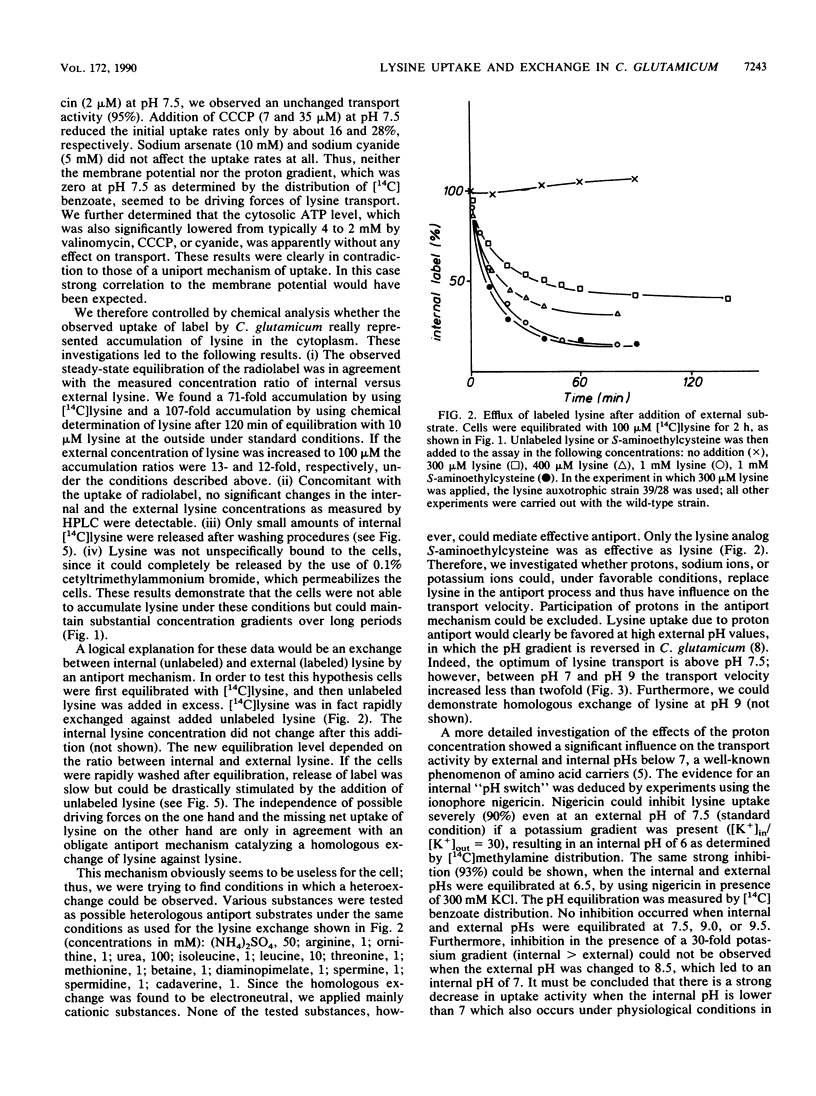
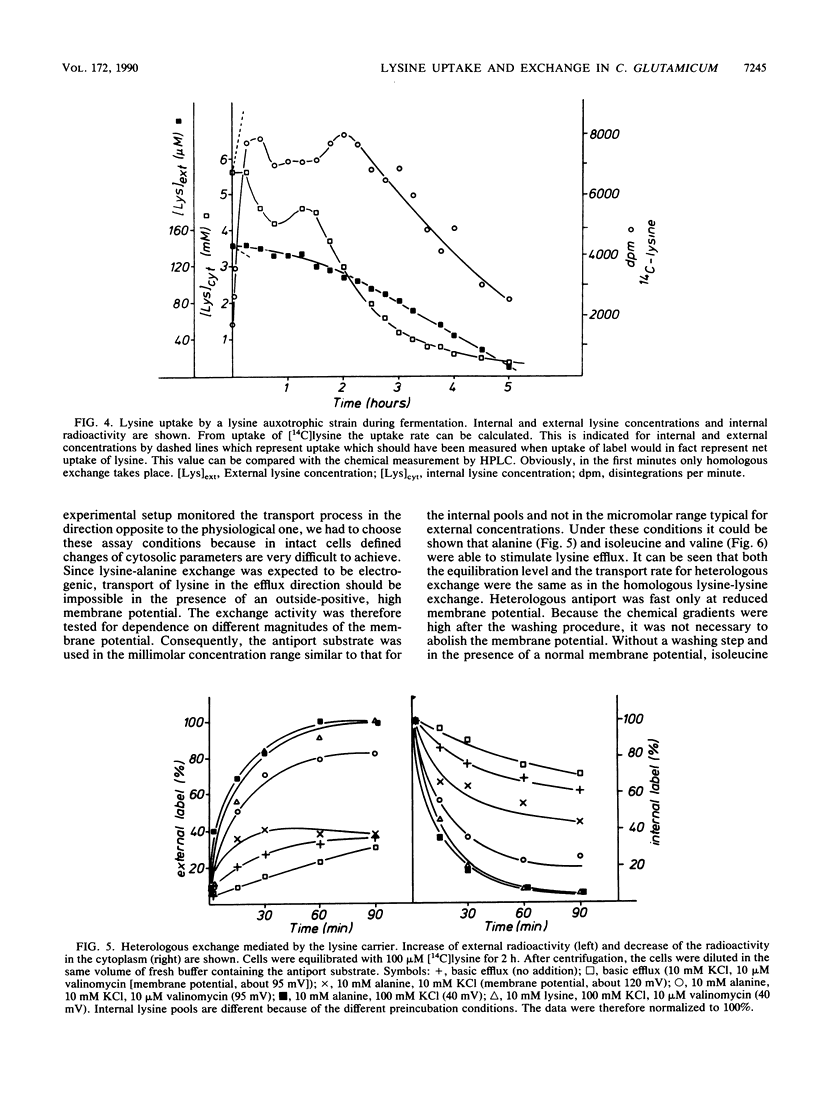
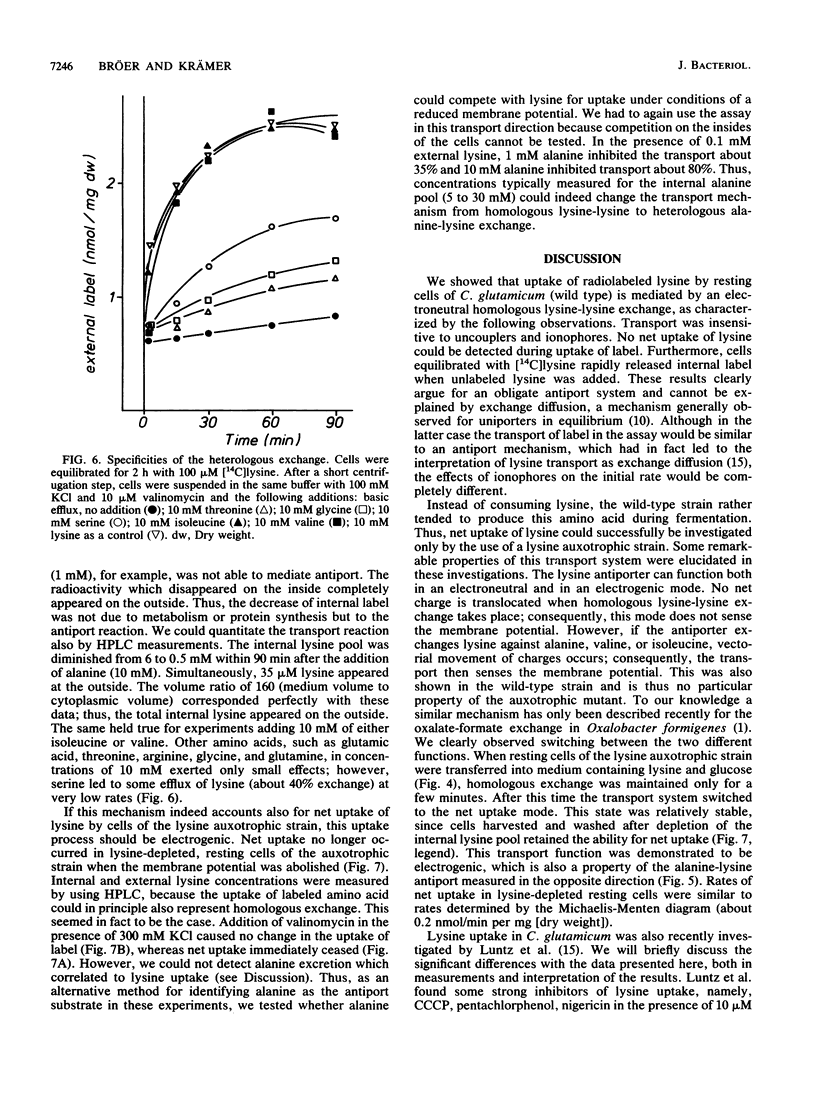
Resting cells of Corynebacterium glutamicum (ATCC 13032) accumulate [14C]lysine by a transport system with a relatively high affinity (10 microMs) and a low maximum velocity (0.15 nmol/min per mg [dry weight]). Uptake of lysine was not inhibited by uncouplers or by ionophores affecting the ion gradients and the energetic state of the cell. Analysis of intracellular amino acid concentrations during the transport reaction as well as kinetic studies revealed that the observed uptake of lysine in fact represents a homologous antiport between extracellular [14C]lysine and intracellular unlabeled lysine. Intracellular [14C]lysine could only be released by the addition of unlabeled lysine to the bacterial suspension. In contrast to this homologous antiport reaction, we observed net uptake of lysine in lysine-depleted cells of a lysine auxotrophic strain. This net uptake was found to be electrogenic and could also be observed as a heterologous antiport reaction in wild-type cells under particular conditions. In this case exchange was mediated between internal lysine and external alanine, isoleucine, or valine. This antiport was electrogenic, since the substrates differ in charge. The cells can switch between electroneutral homologous exchange and electrogenic heterologous antiport mode during fermentation because of changing metabolic conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anantharam V., Allison M. J., Maloney P. C. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J Biol Chem. 1989 May 5;264(13):7244–7250. [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Poolman B., Kiewiet R., Konings W. Arginine transport in Streptococcus lactis is catalyzed by a cationic exchanger. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6093–6097. doi: 10.1073/pnas.84.17.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Smid E. J., Konings W. N. Transport of diamines by Enterococcus faecalis is mediated by an agmatine-putrescine antiporter. J Bacteriol. 1988 Oct;170(10):4522–4527. doi: 10.1128/jb.170.10.4522-4527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., van Leeuwen C., Konings W. N. Transport of basic amino acids by membrane vesicles of Lactococcus lactis. J Bacteriol. 1989 Mar;171(3):1453–1458. doi: 10.1128/jb.171.3.1453-1458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbighausen H., Weil B., Krämer R. Transport of branched-chain amino acids in Corynebacterium glutamicum. Arch Microbiol. 1989;151(3):238–244. doi: 10.1007/BF00413136. [DOI] [PubMed] [Google Scholar]
- Hoischen C., Krämer R. Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J Bacteriol. 1990 Jun;172(6):3409–3416. doi: 10.1128/jb.172.6.3409-3416.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. N., Gilligan J. P. o-Phthaldialdehyde precolumn derivatization and reversed-phase high-performance liquid chromatography of polypeptide hydrolysates and physiological fluids. J Chromatogr. 1983 Aug 26;266:471–482. doi: 10.1016/s0021-9673(01)90918-5. [DOI] [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. Mechanisms of energy coupling to the transport of amino acids by Staphylococcus aureus. Eur J Biochem. 1974 May 15;44(2):517–522. doi: 10.1111/j.1432-1033.1974.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr Mechanism and regulation of phosphate transport in Streptococcus pyogenes. J Bacteriol. 1987 Jan;169(1):297–302. doi: 10.1128/jb.169.1.297-302.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Shiio I., Miyajima R., Kashima N. Na+-dependent transport of threonine in Brevibacterium flavum. J Biochem. 1973 Jun;73(6):1185–1193. doi: 10.1093/oxfordjournals.jbchem.a130190. [DOI] [PubMed] [Google Scholar]
- Thompson J. Ornithine transport and exchange in Streptococcus lactis. J Bacteriol. 1987 Sep;169(9):4147–4153. doi: 10.1128/jb.169.9.4147-4153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Kihara M., Macnab R. M. Measurement of membrane potential in Bacillus subtilis: a comparison of lipophilic cations, rubidium ion, and a cyanine dye as probes. J Membr Biol. 1981;63(3):215–231. doi: 10.1007/BF01870983. [DOI] [PubMed] [Google Scholar]


