Abstract
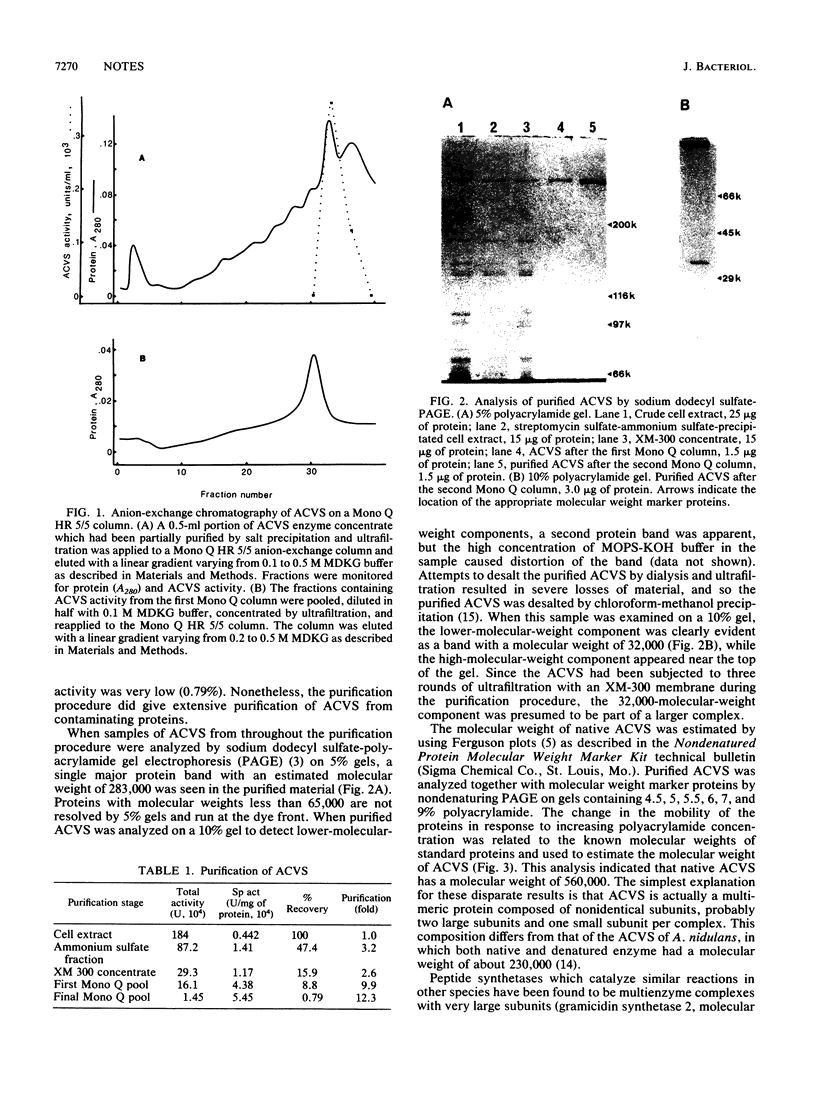
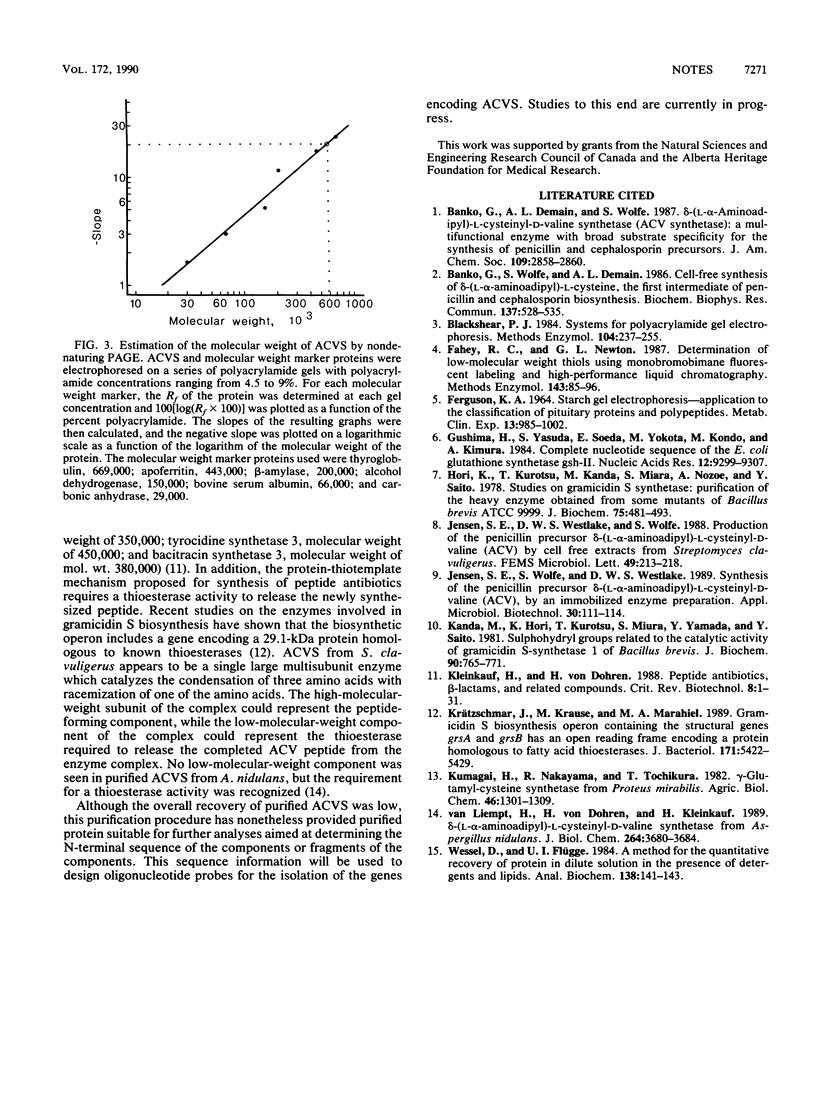
delta-(L-alpha-Aminoadipyl)-L-cysteinyl-D-valine synthetase (ACVS) was purified from Streptomyces clavuligerus by a combination of salt precipitation, ultrafiltration, and anion-exchange chromatography. The final purified material gave two protein bands with molecular weights of 283,000 and 32,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis in nondenaturing gels gave a single protein band with an estimated molecular weight of 560,000. These results suggest that ACVS is a multimer composed of nonidentical subunits.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banko G., Wolfe S., Demain A. L. Cell-free synthesis of delta-(L-alpha-aminoadipyl)-L-cysteine, the first intermediate of penicillin and cephalosporin biosynthesis. Biochem Biophys Res Commun. 1986 May 29;137(1):528–535. doi: 10.1016/0006-291x(86)91242-8. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. Systems for polyacrylamide gel electrophoresis. Methods Enzymol. 1984;104:237–255. doi: 10.1016/s0076-6879(84)04093-3. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Newton G. L. Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- Gushima H., Yasuda S., Soeda E., Yokota M., Kondo M., Kimura A. Complete nucleotide sequence of the E. coli glutathione synthetase gsh-II. Nucleic Acids Res. 1984 Dec 21;12(24):9299–9307. doi: 10.1093/nar/12.24.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M., Hori K., Kurotsu T., Miura S., Yamada Y., Saito Y. Sulfhydryl groups related to the catalytic activity of gramicidin S synthetase 1 of Bacillus brevis. J Biochem. 1981 Sep;90(3):765–771. doi: 10.1093/oxfordjournals.jbchem.a133531. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Peptide antibiotics, beta-lactams, and related compounds. Crit Rev Biotechnol. 1988;8(1):1–32. doi: 10.3109/07388558809150536. [DOI] [PubMed] [Google Scholar]
- Krätzschmar J., Krause M., Marahiel M. A. Gramicidin S biosynthesis operon containing the structural genes grsA and grsB has an open reading frame encoding a protein homologous to fatty acid thioesterases. J Bacteriol. 1989 Oct;171(10):5422–5429. doi: 10.1128/jb.171.10.5422-5429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- van Liempt H., von Döhren H., Kleinkauf H. delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. The first enzyme in penicillin biosynthesis is a multifunctional peptide synthetase. J Biol Chem. 1989 Mar 5;264(7):3680–3684. [PubMed] [Google Scholar]