Abstract
We describe an anion channel in the plasmalemma of protoplasts isolated from wheat (Triticum aestivum L.) roots that is activated by aluminum (Al3+). In the whole-cell configuration, addition of 20–50 μM AlCl3 to the external solution depolarized the membrane and activated an inward current that could remain active for more than 60 min. The activation by Al3+ was rapid in 20% of protoplasts examined, whereas in another 30% a delay of more than 10 min occurred after Al3+ was added. Once the current was activated, changing the external Cl− concentration shifted the membrane reversal potential with ECl, showing that the channel is more selective for anions than cations (Ca2+, K+, tetraethylammonium+). The channel could be activated by Al3+, but not by La3+, and was observed in protoplasts isolated from the root apex but not in protoplasts isolated from mature root tissue. The anion channel antagonist niflumate inhibited the current in whole cell measurements by 83% at 100 μM. Outside-out patch recordings revealed a multistate channel with single-channel conductances of between 27 and 66 pS.
Anion channels participate in a range of important cellular functions in plants, including turgor adjustment, regulation of stomatal aperture, nutrient transport, and the stabilization of membrane potential, Vm (1–3). The large electrical potential difference across the plasmalemma of plant cells (inside negative) means that anion channels usually facilitate anion efflux and therefore provide a means for the rapid depolarization of cell membranes. Because of this, anion channels play a central role in the excitation of plant membranes (3–5). One anion channel has been identified in protoplasts isolated from wheat roots (6). It has a low conductance (about 4 pS) and produces a fast outwardly rectifying current when Vm is more positive than the equilibrium potential for the permeant anion. This channel is a likely pathway for Cl− and NO3− uptake into the root tissue under saline conditions or when an ample supply of NO3− is available (6).
Trivalent cations, such as Al3+ and La3+, are generally toxic to plants, and root growth in wheat is acutely sensitive to micromolar concentrations of these ions. The effects of Al3+ on plants is of particular interest due to its prevalence in soils and ability to severely limit crop and pasture production in acid conditions (7, 8). The sensitivity of plants to Al3+ differs greatly among species, and even genotypes within a species may vary in their ability to grow in Al3+-toxic soils. The mechanisms providing this enhanced tolerance are now becoming clear for some species (8, 9). For instance, in the Al3+-tolerant genotypes of wheat, the presence of Al3+ activates malate and K+ efflux from the root apices (10–12). By chelating Al3+ in the rhizosphere, malate reduces the concentration of these toxic cations near the membrane and mimimizes any harmful interactions with the growing cells at the root apex, a critical zone for Al3+ stress (13). The use of channel antagonists provided evidence that anion channels facilitated the release of malate from those cells (12).
We examined the ionic currents in protoplasts isolated from the apex of Al3+-tolerant wheat roots and describe a novel anion channel that is activated by Al3+ and allows Cl− efflux. This is, to our knowledge, the first report of an ion channel being activated by Al3+. Many properties of this channel are similar to the Al3+-activated efflux of malate from intact roots.
MATERIALS AND METHODS
Plant Material and Protoplast Isolation.
The Al3+-tolerant genotype of wheat (Triticum aestivum L.), designated ET8, was used in these experiments (14). Seeds were surface sterilized with 0.5% NaOCl and grown for 5 to 7 days in 0.2 mM CaCl2, pH 4.5, in a growth cabinet (12 h, 20°C and light/12 h, 22°C and dark, photosynthetic flux density 250 μmol⋅m−2⋅s−1). Protoplasts were prepared from the terminal 2–3 mm of root (root apices) or from the nonapical root tissue, using the procedure described by Schachtman et al. (15).
Solutions.
Protoplasts were placed into a chamber (0.4 ml) with a sealing solution which contained 100 mM KCl and 10 mM CaCl2, pH 6.0. Once the cells had settled a whole-cell configuration was established, as judged by the mean capacitance. The sealing solution was replaced by a control solution that contained a low Ca2+ concentration (0.1–0.5 mM), and either KCl or tetraethylammonium (TEA)Cl (0.2–20 mM) at pH 4.0. A similar solution that contained 20–50 μM AlCl3 was then added. AlCl3 solutions were prepared from a 10 mM stock in 0.1 mM HCl. The use of strong base to adjust the pH of solutions containing Al3+ was avoided to prevent the formation of triskaidekaaluminum (16). Instead, the pH of solutions was raised slightly before the addition of Al3+ and then adjusted down with HCl if required.
The range of pipette solutions used can be grouped into those that contained KCl or TEACl. The base composition for the first group included (in mM): 100 KCl, 10 Hepes, 2 K2ATP, 2 MgCl2, and KOH to pH 7.2. The base composition for the second group included (in mM): 100 TEACl, 10 Hepes, 2 Na2ATP, 2 MgCl2, and TEAOH to pH 7.2. CaCl2 and EGTA were also included in the pipette solutions, and the amounts were varied to manipulate the free Ca2+ concentration from 10 to 689 nM. Sorbitol was used to adjust the osmolality of all bathing solutions to 700 mOsmol/kg and all pipette solutions to 720 mOsmol/kg. The elevated concentrations of Cl− in the pipette solutions allowed the anion currents to be readily observed. Composition of the solutions used are indicated in the figure legends. All solutions were kept at 4°C until required and filtered through a 0.2-μm Millipore filter before use. When required the chemical speciation program geochem (17) was used to calculate the activities of free ions.
Electrophysiology.
Whole-cell and outside-out patch clamp experiments (18) were performed on root protoplasts. The electrical-potential difference across the membrane (Vm) was clamped to different values to construct current–voltage curves. From a holding potential near Erev, the Vm was usually pulsed between approximately −140 mV and +80 mV in 20-mV steps lasting about 5 s. Voltage ramps were also performed on whole cells to produce current–voltage curves and to estimate the variance of current (19). A detailed description of this procedure is given in Results. Single-channel current–voltage curves were obtained from outside-out patches using fast (50-ms) voltage ramps (20). Current measurements were made with a List EPC 7 (List Electronics, Darmstadt, Germany), a Dagan 3900A (Dagan Instruments, Minneapolis) and Axopatch 200A (Axon Instruments, Foster City, CA) patch amplifiers using a Strobes (Strobes Engineering, New Zealand) or P-Clamp6 (Axon Instruments) analysis acquisition systems. Series resistance was compensated to about 50% for all amplifiers and capacitance compensation was possible on the List and Axopatch amplifiers. Single-channel recordings were digitized with a Sony PCM 701 and recorded on video tape. Recorded data were digitized using adcin (J. Pumplin, Department of Physics, Michigan State University, Lansing). Single-channel events were analyzed using the Channel 2 software (Michael Smith, Division of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra). Junction potentials for each change in bathing solution were calculated with the program JPCalc (P. H. Barry, University of New South Wales, Sydney), and the results have been adjusted accordingly. The data are presented using the convention that an inward current (cation influx or anion efflux) is negative.
RESULTS
The protoplasts isolated from the root apices and mature root tissue displayed several different currents in the sealing solution, especially when KCl was included in the pipette solution. These occurred alone or in combination and, in some cases, the three main currents appeared together in a single protoplast. Although these currents were not examined in detail they were similar to those already described for mature root cells. These include a time-dependent outward and a time-dependent inward current, characteristic of the K+-outward and K+-inward rectifiers (15, 21, 22) and a fast, outwardly rectifying anion current (6).
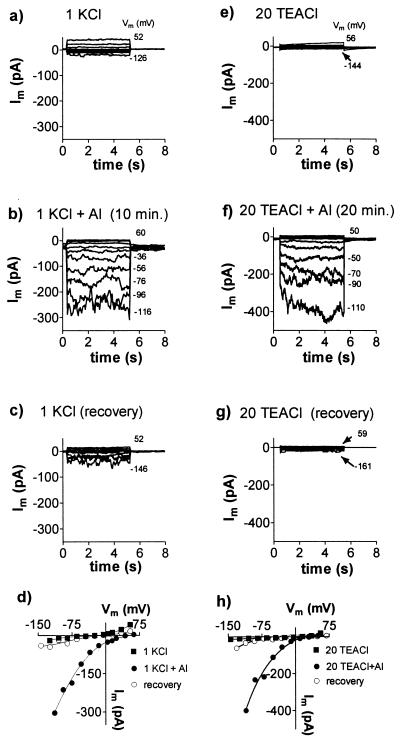
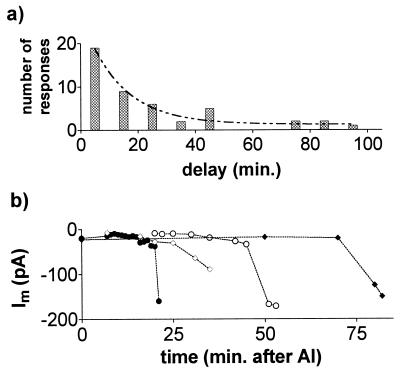
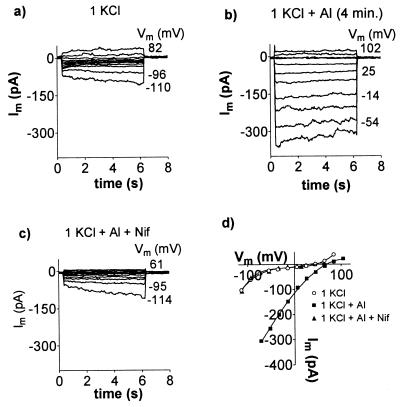
In experiments designed to test the effects of Al3+, the sealing solution was first replaced by a low-CaCl2, low-pH control solution and then replaced by an identical solution that contained AlCl3 (20–50 μM). The initial effects of Al3+ on the whole cell currents varied. In 20% of protoplasts examined from the root apex (n = 73) the addition of Al3+ immediately shifted the reversal potential (Erev) in the positive direction and activated an inward current. In a further 30% of protoplasts, the membrane conductance was initially reduced by Al3+ and an inward current was activated after a delay. In the remainder of the cells Al3+ either had no effect or inhibited the currents. The results were similar whether KCl or TEACl was used in the pipette solution. Fig. 1 shows inward current activated by Al3+ in whole cells. When Al3+ was removed from the chamber by rinses with control solution, the inward current decreased and Erev shifted back in the negative direction (Fig. 1 c and g). The histogram in Fig. 2a shows the distribution of times required for Al3+ to activate the inward current. The data are fitted with an exponential curve with a time constant of 13.47 min which is also the mean response time. Fig. 2b shows the increase in whole-cell current through time from four cells. The reason for this delay is unclear, but it was observed with a range of free Ca2+ concentrations in the pipette (10–689 nM). Furthermore, the addition of 100 μM guanosine 5′-[γ-thio]triphosphate or 100 μM inositol 1,4,5-trisphosphate (IP3) to the pipette solution, or the exclusion of ATP, had no consistent effects (not shown). Once activated, the inward current would often remain active for more than 60 min. By contrast, when protoplasts isolated from the mature root tissue were treated with Al3+ the membrane conductance was consistently reduced. On no occasion did Al3+ activate an inward current in these protoplasts even after extended treatment times (not shown).
Figure 1.
Activation of an inward current in whole cells by AlCl3. Results are shown from two experiments which included either KCl (a–d) or TEACl (e–h) in the solutions. Whole-cell currents, Im, were recorded as Vm was stepped from a holding potential near 0 mV in 20-mV intervals to the levels shown (to the right of the current traces). Plots a–c and e–g represent a sequence of solution changes. The control solution shown in a comprised 1 mM KCl and 0.2 mM CaCl2, pH 4.0. The control solution shown in e contained 20 mM TEACl and 0.2 mM CaCl2, pH 4.0. Similar solutions containing 50 μM AlCl3 were added in b and f, and the currents were recorded after the time indicated. AlCl3 was then removed by changing to the control solution again as shown in c and g. Currents measured at the end of the voltage pulses were used to construct the current–voltage curves shown in d and h. The pipette solution for a–d was (mM) 90 KCl, 7.6 CaCl2, 10 EGTA, 10 Hepes, and 48 KOH to pH 7.2. The pipette solution for e–h was (mM) 100 TEACl, 2 MgCl2, 10 Hepes, 2 EGTA, 2 Na2ATP, and 20 TEAOH to pH 7.2.
Figure 2.
Time-dependent activation of whole-cell currents by Al3+. (a) Frequency histogram showing the time required, after addition of Al3+, to activate the inward current. Columns indicate activation occurring between 0 and 10 min or between 11 and 20 min, etc. Data are fitted with an exponential curve. (b) Time-dependent increase of inward currents measured at −90 mV after addition of Al3+. Results from four different experiments are shown.
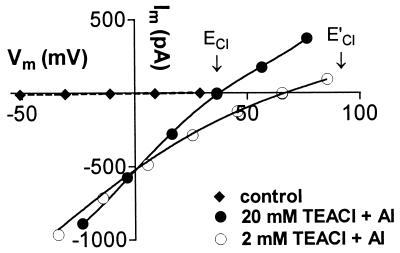
After the inward current had been activated by Al3+ in whole cells, changing the external Cl− concentrations consistently shifted Erev with ECl, indicating that Cl− efflux was contributing more to the inward current than the influx of the cations present (Ca2+ and K+ or TEA+). Fig. 3 shows Erev shift from 38 mV to 66 mV when the external TEACl concentration was changed from 20 mM to 2 mM. This was observed whether the concentration of Al3+ was kept constant or adjusted in each solution to maintain the activity of the free Al3+ cations constant (see Fig. 3). The mean shift measured in nine experiments was 33 ± 5 mV (±SE). In eight of nine experiments the whole-cell inward current decreased when the external solution was changed from 20 TEACl to 2 mM TEACl although Erev shifted in the positive direction.
Figure 3.
Effect of external Cl− concentration on whole-cell currents. All external solutions contained 0.1 mM CaCl2, pH 4.0, with the following additions: ⧫, 20 mM TEACl; •, 20 mM TEACl, 50 μM AlCl3; ○, 2 mM TEACl, 25 μM AlCl3. Arrows indicate the ECl for each solution (E′Cl for 2 mM TEACl). Pipette solution (mM): 100 TEACl, 2 MgCl2, 2 Na2ATP, 2 EGTA, 10 Hepes, 20 TEAOH to pH 7.2. The activity of free Al3+ cations was 14 μM in both solutions.
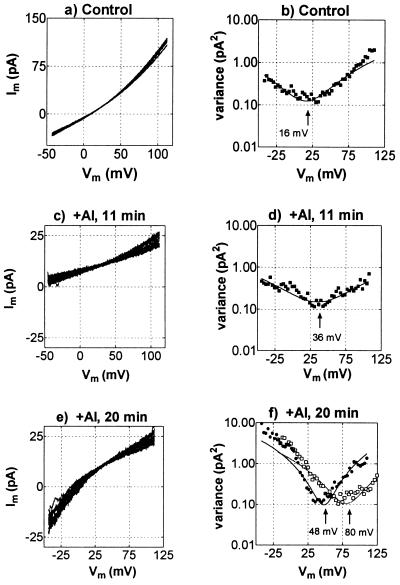
Identity of the current was confirmed by analyzing voltage ramps performed on the whole-cell membrane during this response to Al3+. Fig. 4 a, c, and e shows the current–voltage curves measured before addition of Al3+ and 11 min and 20 min after the addition of 50 μM Al3+. The reversal potential of the voltage ramps will depend on various channels and transporters in the membrane. However, by obtaining the variance of the currents between ramps and plotting this as a function of voltage it is possible to obtain an estimate of the reversal potential of the dominant ion channels in the membrane (Fig. 4 b, d, and f). This depends on the current variance reaching a minimum when the voltage is at the reversal potential of an active channel. In the control curves (Fig. 4 a and b) the Erev is about 16 mV, which suggests the presence of a nonselective cation channel permeable to TEA+ and or Ca2+. These channels were inhibited by Al3+, causing a reduction in membrane conductance and reduced variance (Fig. 4 c and d). The negative shift in Erev indicates that the proton pump dominated the conductance when the channels were inhibited by Al3+, but the positive shift of the minimum from the variance curve is consistent with an increase in Cl− conductance (Fig. 4d). After 20 min the curves shifted more positive, corresponding to the increased activation of an inward current (Fig. 4 e and f). The variance increased at negative voltages and the minimum from the variance plot is close to ECl (Fig. 4f). When the external TEACl concentration was reduced from 20 mM to 2 mM the minimum in variance shifted with ECl (Fig. 4f).
Figure 4.
Current–voltage curves and variance–voltage curves derived from voltage ramps in the whole-cell configuration before and after addition of 50 μM AlCl3. For each treatment 15 sequential ramps, lasting 250 ms each, were applied between about −45 and 110 mV (2-kHz sampling, 0.5-kHz, four-pole Bessel filter). These are shown superimposed in each of a, c, and e. To the right of each current–voltage curve in b, d, and f is shown the variance–voltage curve, and the minima were obtained by fitting quadratic functions to the data. f also shows the variance–voltage curve when TEACl was reduced from 20 mM to 2 mM (□ and dotted line). Pipette solution (mM): 100 TEACl, 2 EGTA, 10 Hepes, 7 TEAOH to pH 7.2. The bath solution consisted of (mM) 20 TEACl, 0.2 CaCl2, pH 4.
The Al3+-activated currents were inhibited by the anion channel antagonist niflumate (Fig. 5). At a holding potential of −100 mV, the whole-cell current was inhibited by 62% ± 8% with 50 μM niflumate and by 83% ± 6% with 100 μM niflumate (n = 3). The inhibition was reversible in some experiments (see Fig. 5), while in others several rinses with a solution containing Al3+ without niflumate failed to reactivate the currents.
Figure 5.
Effect of niflumate on the Al3+-activated anion current. Whole-cell currents were recorded as Vm was stepped in 20-mV intervals to the levels shown. Plots a, b, and c represent sequential solution changes, and the current–voltage values at the end of the pulses are shown in d. External solutions contained 1 mM KCl and 0.5 mM CaCl2, pH 4.2, with the following additions: b, 50 μM AlCl3; c, 50 μM AlCl3 and 100 μM niflumate (Nif). Pipette solution (mM): 90 KCl, 7.6 CaCl2, 10 EGTA, 10 Hepes, 2 MgCl2, 2 K2ATP, and 50 KOH to pH 7.2.
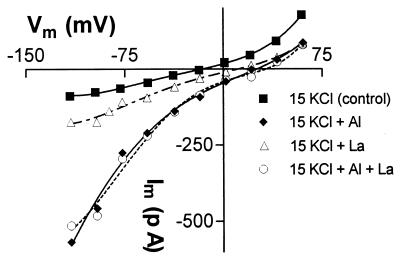
We tested whether a different trivalent cation could activate the inward current. When La3+ was applied to previously untreated protoplasts (in the KCl solutions), the inward and outward currents were either unchanged or inhibited (n = 4). Since it is possible that insufficient time was allowed for La3+ to activate the Cl− currents, Al3+ was added first to activate the inward current and then it was replaced with La3+. On all occasions (n = 8) the Al3+-activated current was diminished (see Fig. 6). Either the current was deactivating when Al3+ was removed from the external solution or La3+ was blocking the Al3+-activated currents directly. This was tested with the following protocol: (i) Al3+ was first added to activate the Cl− current, (ii) Al3+ was replaced by La3+ in the external solution, and (iii) Al3+ and La3+ were added together. On all occasions (n = 3) the current diminished when Al3+ was replaced by La3+ but the current reactivated in the combined Al3+ + La3+ treatment (see Fig. 6). These results indicate that La3+ cannot replace Al3+ in activating the current and that La3+ does not block the channels mediating Cl− efflux.
Figure 6.
Effect of La3+ on the Al3+-activated anion current. Current–voltage curves were measured in whole-cell membranes in the treatments shown (in sequence). All external solutions contained 15 mM KCl and 0.5 mM CaCl2, pH 4.2 (control, ▪) with the following additions: ⧫, 50 μM AlCl3; ▵, 50 μM LaCl3; and ▪, 50 μM AlCl3 plus 50 μM LaCl3. Pipette solution (mM): 70 KCl, 2.3 CaCl2, 10 EGTA, 10 Hepes, 2 MgCl2, 2 K2ATP, and 35 KOH to pH 7.2.
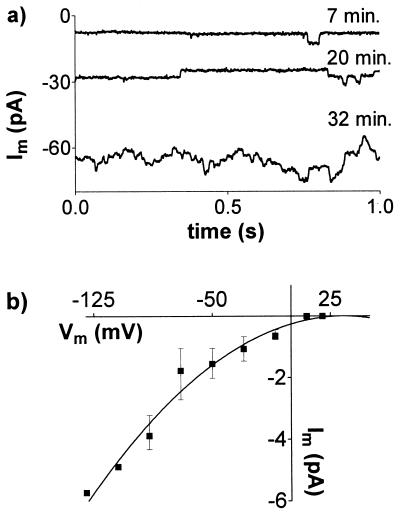
The conductance of the whole-cell membrane in the TEACl solutions was often low enough for single-channel events to be detected. Fig. 7a shows the time-dependent increase of single-channel activity recorded in a whole cell after addition of Al3+. Similar data were collected from six protoplasts, and the current–voltage relationship for the single-channel events is shown in Fig. 7b.
Figure 7.
Single-channel recordings from whole cells. (a) Single-channel activity at 7, 20, and 32 min after adding AlCl3. Currents were measured at −90 mV. (b) Current–voltage relationship for channels activated by Al3+ (mean ± SE, n = 6). External solution contained 20 mM TEACl, 0.2 mM CaCl2, and 50 μM AlCl3, pH 4.0. See Fig. 3 for pipette solution.
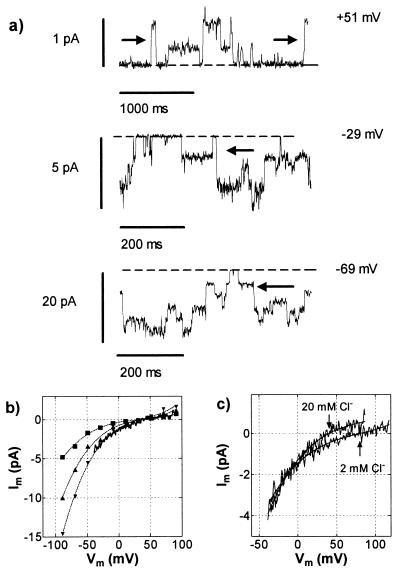
Activation of the anion channel by Al3+ was recorded only in the whole-cell configuration, and attempts to activate the response in detached, outside-out patches were unsuccessful. However single-channel events could be observed in outside-out patches excised from whole cells after the inward current had been activated by Al3+. Application of fast (50-ms) depolarizing voltage ramps to these outside-out patches generated currents with voltage dependence similar to those observed in whole cells (Fig. 8b). At first sight, the patch yielding the data in Fig. 8 appeared to contain several channels of equal conductance, but analysis of the data showed that the channels were not independent, because transitions in current were not confined to adjacent levels. The arrows in Fig. 8a show direct transitions between more than one level. Furthermore, amplitude histograms of the data (not shown) showed that the difference between the closed state and the first open level was greater than between subsequent levels. The chord conductance of the most common state was 27 pS at 0 mV and 66 pS at −50 mV. When the external TEACl was reduced from 20 mM to 2 mM, Erev shifted in the positive direction, confirming that the channel was permeable to Cl− (Fig. 8c).
Figure 8.
Al3+-induced currents through an outside-out patch. (a) Current at three levels of Vm. The arrows indicate transitions in current of magnitude greater than that between individual levels. Broken lines show closed state of the channel. (b) Current–voltage curves obtained from mean amplitude histograms of the current amplitudes. A current–voltage curve obtained from a voltage ramp is superimposed. (c) Current–voltage curves obtained from voltage ramps in 20 mM and 2 mM TEACl. Pipette solution (mM): 100 TEACl, 2 EGTA, 10 Hepes, and 7 TEAOH to pH 7.2. The external solution consisted of 20 mM TEACl, 0.2 mM CaCl2, and 50 μM Al3+, pH 4, for the high-Cl− solution and 2 mM TEACl, 0.2 mM CaCl2, and 25 μM Al3+, pH 4, for the low-Cl− solution. The activity of free Al3+ cations was 13.5 μM in both solutions.
The time-dependent inward and outward K+ currents which were observed when KCl was present in the sealing and control solutions were sensitive to Al3+. The time-dependent inward current was present in 44% (n = 36) of protoplasts and inhibited by AlCl3 with an IC50 of 40 μM (n = 6), which corresponded to an activity for free Al3+ ions of 12 μM. The time-dependent outward current was observed in 36% of protoplasts and was inhibited by AlCl3 with an IC50 of 25 μM (n = 8), which corresponded to an activity for free Al3+ ions of 7 μM.
DISCUSSION
We have characterized a novel anion channel from wheat roots that is activated by Al3+ and allows anion efflux. The activation of ion channels by cations has been reported in some animal cells (23, 24), but as far as we know this is the first report of a channel being activated by Al3+. Indeed, Al3+ has been shown to be a potent antagonist of many channels, including an inward-rectifying K+ channel in wheat roots (22), a Ca2+ channel in wheat roots (25, 26), a mechanosensory Ca2+ channel in onion (27), and the VDAC channel from mitochondria (28). We have confirmed the sensitivity of the K+ inward-rectifying channel to Al3+ and further report that the time-dependent, outward-rectifying K+ current is also blocked by Al3+ with an IC50 of 25 μM.
The contribution of Cl− efflux to the inward current was confirmed in whole-cell and single-channel measurements by the shifts in Erev with different Cl− concentrations (Figs. 3 and 8). During these experiments we noticed that the whole-cell membrane conductance and single-channel conductance decreased when the external Cl− concentration was lowered. A similar response was observed in guard cells where an increase in external Cl− concentration raised the single-channel conductance of the GCACl anion channel (29). It was suggested that this positive-feedback response would maintain an efflux of anions from guard cells during stomatal closure despite the increasing anion concentration outside the cell and the diminished gradient across the membrane. It could be similarly argued here that this feedback could sustain an efflux of anions in the presence of Al3+ despite a diminishing gradient across the membrane. Once activated by Al3+, the Cl− current could remain active for more than 60 min if Al3+ remained in the solution. In this respect the Al3+-activated Cl− channel is similar to the S-type anion conductance from guard cells. That channel displays little or no inactivation, allowing it to facilitate the sustained release of ions required for stomatal closure (30). Anion channels displaying similarities with the S-type channels have also been described in Nicotiana suspension cells (31), xylem parenchyma from Hordeum roots (32), and Arabidopsis hypocotyls (33, 34).
The activation of inward current by Al3+ occurred without any discernible delay in almost half of the protoplasts that showed a response, while others showed a lag of 10–90 min (Fig. 2). The exponential distribution between time for activation and frequency of responses suggests that activation might involve a probabilistic determinant. For example, if activation requires a receptor or channel to move into a “receptive” state before it can interact with Al3+, then an exponential distribution, such as is shown in Fig. 2a, would be expected. However some of the delays reported here are exceptional even compared with other plant channels with long activation times (33, 35, 36). The voltage regulation and time dependence of ion channels can be modulated by a range of cytoplasmic factors, including nucleotides, Ca2+ concentration, GTP-binding proteins, IP3, hormones, and kinases (31, 33, 37–42). For example, the activation of an anion channel by polyvalent cations in bovine parathyroid cells requires a membrane-bound receptor and a secondary-messenger cascade involving a rise in cytoplasmic Ca2+ (23). However, in the whole-cell configuration the cytoplasm is replaced by the pipette solution, disturbing any secondary-messenger pathways involving soluble cytoplasmic components. Therefore the presence of a delay might also indicate an impaired signal pathway, and failure of Al3+ to activate the current in outside-out patches is consistent with this idea. In the present study we found that changing the concentration of ATP, guanosine 5′-[γ-thio]triphosphate, IP3, or Ca2+ in the pipette (cytoplasm) solution had no effect on this response, and in future studies we will investigate whether other components are involved.
Lanthanum was used to test for any nonspecific effects of trivalent cations (Fig. 6). We demonstrated that La3+ could not activate the anion current in the same manner as Al3+. Furthermore, La3+ does not block the Al3+-activated Cl− current, which contrasts with the inhibitory effects of La3+ on another Cl− conductance in the alga Chara inflata (43).
Many characteristics of the Al3+-activated anion channel described here are similar to those of the Al3+-dependent efflux of malate observed from intact roots of Al3+-tolerant wheat plants (10, 12). For instance, malate efflux was found to be localized to the root apex, and other trivalent cations tested (including La3+) were unable to substitute for Al3+ in activating the response. This is consistent with the observation that Al3+-tolerant wheat plants are not tolerant of La3+. Malate efflux continued for as long as the roots were exposed to Al3+ (>24 h) and the response was inhibited by niflumate, 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB), and [(6,7dichloro-2-cyclopentyl-2,3-dihydro-2-methyl-oxo-1H-inden5-yl)oxy]acetic acid (IAA-94), but not by La3+ or 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS). A notable difference between that study and the present work is that anion efflux from roots was activated immediately by Al3+, which was not always the case here, as discussed above. We have yet to establish whether the Al3+-activated anion channel is permeable to organic anions, but other anion channels characterized in plant cells do show some permeability to malate ions (29, 44–46).
Aluminum was found to be an effective antagonist of the outward-rectifying K+ channel, indicating that this channel is unlikely to facilitate the K+ release which accompanies malate efflux from Al3+-treated wheat roots (12). An additional cation conductance must be activated to account for the K+ efflux in intact roots. Without examining the matter in detail, we found indications in the present work that another current was being activated by Al3+. For instance, Al3+ occasionally increased the whole-cell conductance without significantly shifting Erev, which remained between ECl and EK. Aluminum appeared to be activating both inward and outward currents in these cells. These extra conductances will be examined in the future to determine whether they can facilitate K+ efflux when the outward-rectifying K+ channel is blocked by Al3+. Nonspecific cation channels may be involved, and these have been described in a range of plant cells (see ref. 47).
We have described a novel anion channel from wheat roots that is activated by Al3+ but not La3+ and allows anion efflux. The localization of the channel to protoplasts isolated from the root apex, its sensitivity to niflumate, and its sustained activity are consistent with this channel mediating the Al3+-activated malate efflux observed in Al3+-tolerant wheat roots.
Acknowledgments
We are grateful to Ashley Garrill for an early involvement in this project and to Dianne Trussell for technical assistance. This work was supported by an Australian Research Grant to S.D.T. and G.P.F.
ABBREVIATIONS
- ECl and EK
Nernst equilibrium potential differences for Cl− and K+, respectively
- Erev
membrane reversal potential
- IP3
inositol 1,4,5-trisphosphate
- TEA
tetraethylammonium
- Vm
electrical potential difference across the plasmalemma
References
- 1.Tyerman S D. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:351–373. [Google Scholar]
- 2.Schroeder J I. Plant Mol Biol. 1995;28:353–361. doi: 10.1007/BF00020385. [DOI] [PubMed] [Google Scholar]
- 3.Ward J M, Pel Z-M, Schroeder J I. Plant Cell. 1995;7:833–844. doi: 10.1105/tpc.7.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaffey C T, Mullins J L. J Physiol. 1958;144:505–524. doi: 10.1113/jphysiol.1958.sp006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hope A B, Findlay G P. Plant Cell Physiol. 1964;5:377–379. [Google Scholar]
- 6.Skerrett M, Tyerman S D. Planta. 1994;192:295–305. [Google Scholar]
- 7.Taylor G J. Metal Ions in Biological Systems: Aluminum and Its Role in Biology. Vol. 24. New York: Dekker; 1988. pp. 165–198. [Google Scholar]
- 8.Kochian L V. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- 9.Delhaize E, Ryan P R. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delhaize E, Ryan P R, Randall P J. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu U, Godbold D, Taylor G J. J Plant Physiol. 1994;144:747–753. [Google Scholar]
- 12.Ryan P R, Delhaize E, Randall P J. Planta. 1995;196:103–110. [Google Scholar]
- 13.Ryan P R, DiTomaso J M, Kochian L V. J Exp Bot. 1993;44:437–446. [Google Scholar]
- 14.Delhaize E, Craig S, Beaton C D, Bennet R J, Jagadish V C, Randall P J. Plant Physiol. 1993;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schachtman D P, Tyerman S D, Terry B R. Plant Physiol. 1991;97:598–605. doi: 10.1104/pp.97.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinraide T B. Plant Soil. 1992;134:167–178. [Google Scholar]
- 17.Parker D R, Zelazny L W, Kinraide T B. Soil Sci Soc Am J. 1987;51:488–491. [Google Scholar]
- 18.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann S H, Conti F. Methods Enzymol. 1992;207:131–148. doi: 10.1016/0076-6879(92)07009-d. [DOI] [PubMed] [Google Scholar]
- 20.Tyerman S D, Findlay G P. J Exp Bot. 1989;40:105–117. [Google Scholar]
- 21.Findlay G P, Tyerman S D, Garrill A, Skerrett M. J Membr Biol. 1994;139:103–116. doi: 10.1007/BF00232429. [DOI] [PubMed] [Google Scholar]
- 22.Gassmann W, Schroeder J I. Plant Physiol. 1994;105:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown E M, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M A, Lytton J, Herbert S C. Nature (London) 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 24.Riccardi D, Park J, Lee W-S, Gamba G, Brown E M, Hebert S C. Proc Natl Acad Sci USA. 1995;92:131–135. doi: 10.1073/pnas.92.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J-W, Pellet D M, Papernik L A, Kochian L V. Plant Physiol. 1994;110:561–569. doi: 10.1104/pp.110.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piñeros M, Tester M. Planta. 1995;195:478–488. [Google Scholar]
- 27.Ding J P, Badot P-M, Pickard B G. Aust J Plant Physiol. 1993;20:771–778. doi: 10.1071/pp9930771. [DOI] [PubMed] [Google Scholar]
- 28.Dill E T, Holden M J, Colombini M. J Membr Biol. 1987;99:187–196. doi: 10.1007/BF01995699. [DOI] [PubMed] [Google Scholar]
- 29.Hedrich R, Marten I. EMBO J. 1993;12:897–901. doi: 10.1002/j.1460-2075.1993.tb05730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder J I, Schmidt C, Sheaffer J. Plant Cell. 1993;5:1831–1841. doi: 10.1105/tpc.5.12.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann S, Thomine S, Guern J, Barbier-Brygoo H. Plant J. 1994;6:707–716. [Google Scholar]
- 32.Wegner L H, Rasche K. Plant Physiol. 1994;105:799–813. doi: 10.1104/pp.105.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomine S, Zimmermann S, Guern J, Barbier-Brygoo H. Plant Cell. 1995;7:2091–2100. doi: 10.1105/tpc.7.12.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho M H, Spalding E. Proc Natl Acad Sci USA. 1996;93:8134–8138. doi: 10.1073/pnas.93.15.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linder B, Rasche K. FEBS Lett. 1992;313:27–30. doi: 10.1016/0014-5793(92)81176-m. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder J I, Keller B U. Proc Natl Acad Sci USA. 1992;89:5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietrich P, Hedrich R. Planta. 1994;195:301–304. [Google Scholar]
- 38.Schroeder J I, Hagiwara S. Nature (London) 1989;338:427–430. [Google Scholar]
- 39.Alexandre J, Lassalles J P, Kado R T. Nature (London) 1990;343:567–570. [Google Scholar]
- 40.Hedrich R, Busch H, Rasche K. EMBO J. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fairley-Grenot K, Assmann S M. Plant Cell. 1991;3:1037–1044. doi: 10.1105/tpc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly W B, Esser J E, Schroeder J I. Plant J. 1995;8:479–489. [Google Scholar]
- 43.Tyerman S D, Findlay G P, Paterson G J. J Membr Biol. 1986;89:153–161. [Google Scholar]
- 44.Pantoja O, Gelli A, Blumwald E. Plant Physiol. 1992;100:1137–1141. doi: 10.1104/pp.100.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt C, Schroeder J I. Plant Physiol. 1994;106:383–391. doi: 10.1104/pp.106.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerana R, Giromini L, Colombo R. Aust J Plant Physiol. 1995;22:115–121. [Google Scholar]
- 47.Tyerman, S. D., Skerrett, M., Garrill, A., Findlay, G. P. & Leigh, R. A. (1997) J. Exp. Bot., in press. [DOI] [PubMed]