Abstract
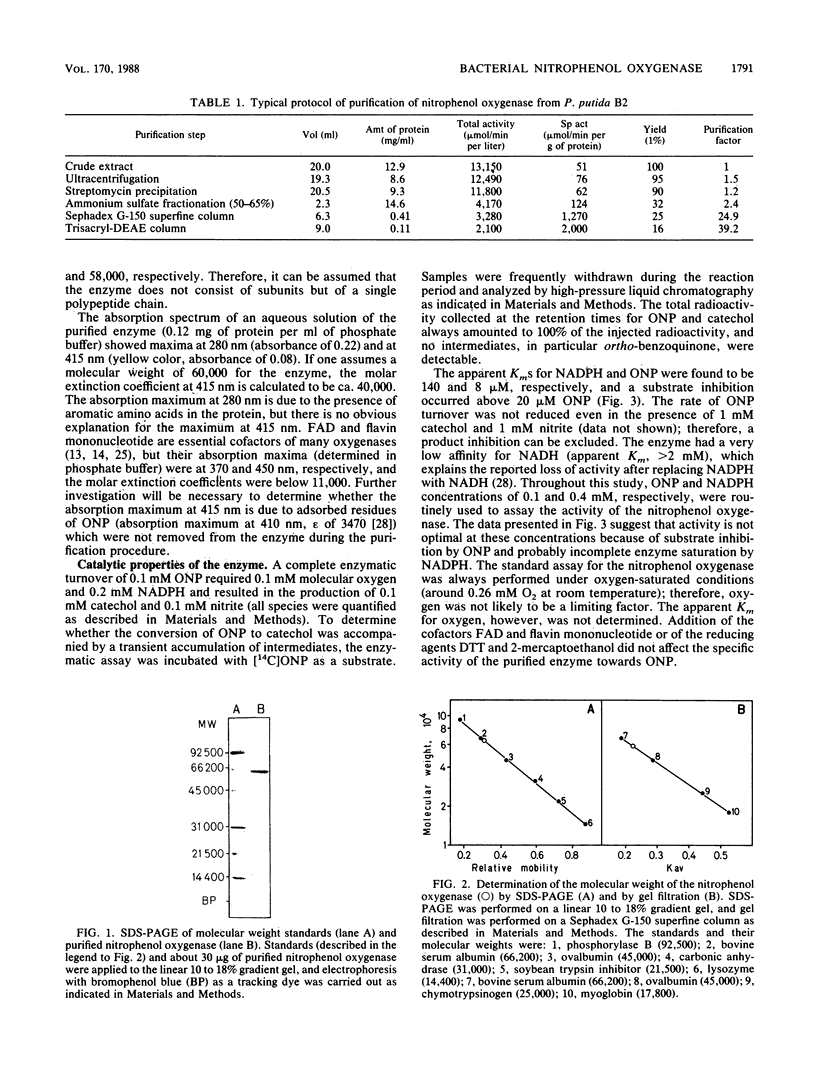
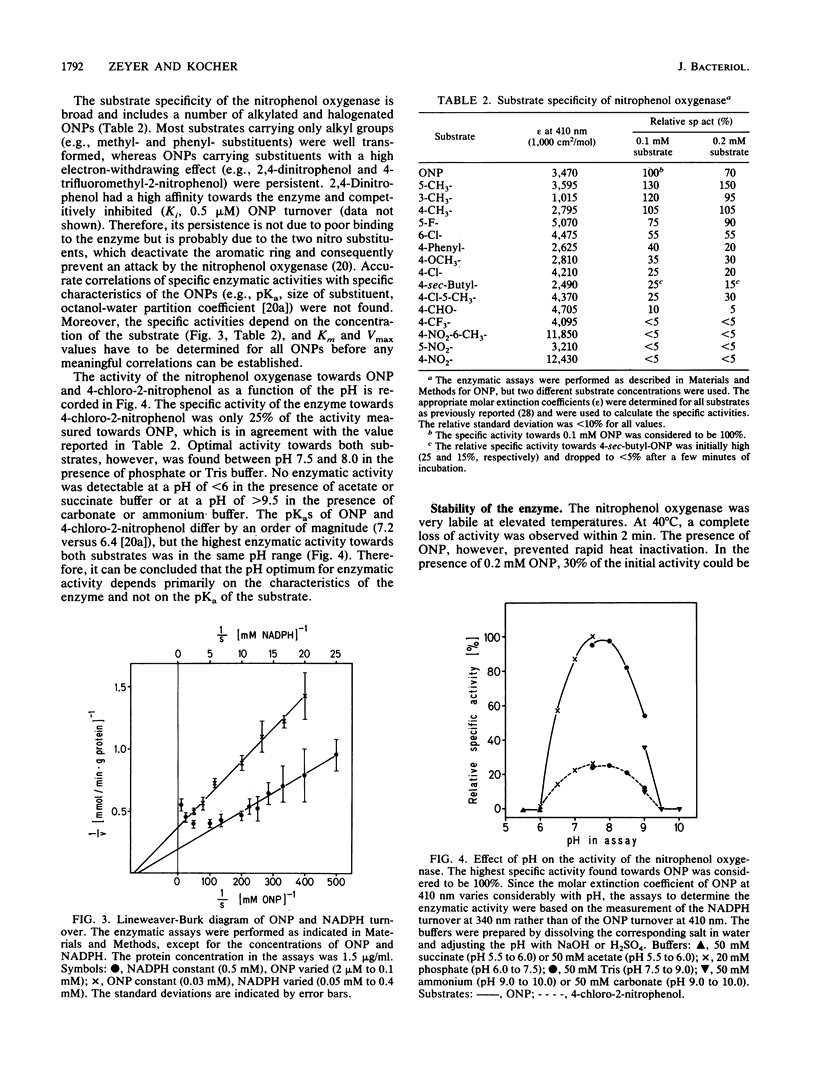
A nitrophenol oxygenase which stoichiometrically converted ortho-nitrophenol (ONP) to catechol and nitrite was isolated from Pseudomonas putida B2 and purified. The substrate specificity of the enzyme was broad and included several halogen- and alkyl-substituted ONPs. The oxygenase consisted of a single polypeptide chain with a molecular weight of 58,000 (determined by gel filtration) or 65,000 (determined on a sodium dodecyl sulfate-polyacrylamide gel). The enzymatic reaction was NADPH dependent, and one molecule of oxygen was consumed per molecule of ONP converted. Enzymatic activity was stimulated by magnesium or manganese ions, whereas the addition of flavin adenine dinucleotide, flavin mononucleotide, or reducing agents had no effect. The apparent Kms for ONP and NADPH were 8 and 140 microM, respectively. 2,4-Dinitrophenol competitively (Ki = 0.5 microM) inhibited ONP turnover. The optimal pH for enzyme stability and activity was in the range of 7.5 to 8.0. At 40 degrees C, the enzyme was totally inactivated within 2 min; however, in the presence of 1 mM ONP, 40% of the activity was recovered, even after 10 min. Enzymatic activity was best preserved at -20 degrees C in the presence of 50% glycerol.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARTWRIGHT N. J., CAIN R. B. Bacterial degradation of the nitrobenzoic acids. Biochem J. 1959 Feb;71(2):248–261. doi: 10.1042/bj0710248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERMANIER R., WUHRMANN K. UBER DEN AEROBEN MIKROBIELLEN ABBAU AROMATISCHER NITROVERBINDUNGEN. Pathol Microbiol (Basel) 1963;26:569–578. [PubMed] [Google Scholar]
- Hallas L. E., Alexander M. Microbial transformation of nitroaromatic compounds in sewage effluent. Appl Environ Microbiol. 1983 Apr;45(4):1234–1241. doi: 10.1128/aem.45.4.1234-1241.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T., Hashizume K., Soda K. Purification and properties of nitroalkane oxidase from Fusarium oxysporum. J Bacteriol. 1978 Jan;133(1):53–58. doi: 10.1128/jb.133.1.53-58.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido T., Soda K., Suzuki T., Asada K. A new oxygenase, 2-nitropropane dioxygenase of Hansenula mrakii. Enzymologic and spectrophotometric properties. J Biol Chem. 1976 Nov 25;251(22):6994–7000. [PubMed] [Google Scholar]
- Kinouchi T., Ohnishi Y. Purification and characterization of 1-nitropyrene nitroreductases from Bacteroides fragilis. Appl Environ Microbiol. 1983 Sep;46(3):596–604. doi: 10.1128/aem.46.3.596-604.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCormick N. G., Feeherry F. E., Levinson H. S. Microbial transformation of 2,4,6-trinitrotoluene and other nitroaromatic compounds. Appl Environ Microbiol. 1976 Jun;31(6):949–958. doi: 10.1128/aem.31.6.949-958.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Wyss O., Gibson D. T. Enzymatic oxidation of p-nitrophenol. Biochem Biophys Res Commun. 1979 May 28;88(2):634–641. doi: 10.1016/0006-291x(79)92095-3. [DOI] [PubMed] [Google Scholar]
- Terada H. The interaction of highly active uncouplers with mitochondria. Biochim Biophys Acta. 1981 Dec 30;639(3-4):225–242. doi: 10.1016/0304-4173(81)90011-2. [DOI] [PubMed] [Google Scholar]
- VILLANUEVA J. R. THE PURIFICATION OF A NITRO-REDUCTASE OF NOCARDIA V. J Biol Chem. 1964 Mar;239:773–776. [PubMed] [Google Scholar]
- White-Stevens R. H., Kamin H., Gibson Q. H. Studies of a flavoprotein, salicylate hydroxylse. I. Enzyme mechanism. J Biol Chem. 1972 Apr 25;247(8):2371–2381. [PubMed] [Google Scholar]
- Zeyer J., Kocher H. P., Timmis K. N. Influence of para-substituents on the oxidative metabolism of o-nitrophenols by Pseudomonas putida B2. Appl Environ Microbiol. 1986 Aug;52(2):334–339. doi: 10.1128/aem.52.2.334-339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenhorn J., Senn M., Bücher T. Molar absorptivities of beta-NADH and beta-NADPH. Clin Chem. 1976 Feb;22(2):151–160. [PubMed] [Google Scholar]



