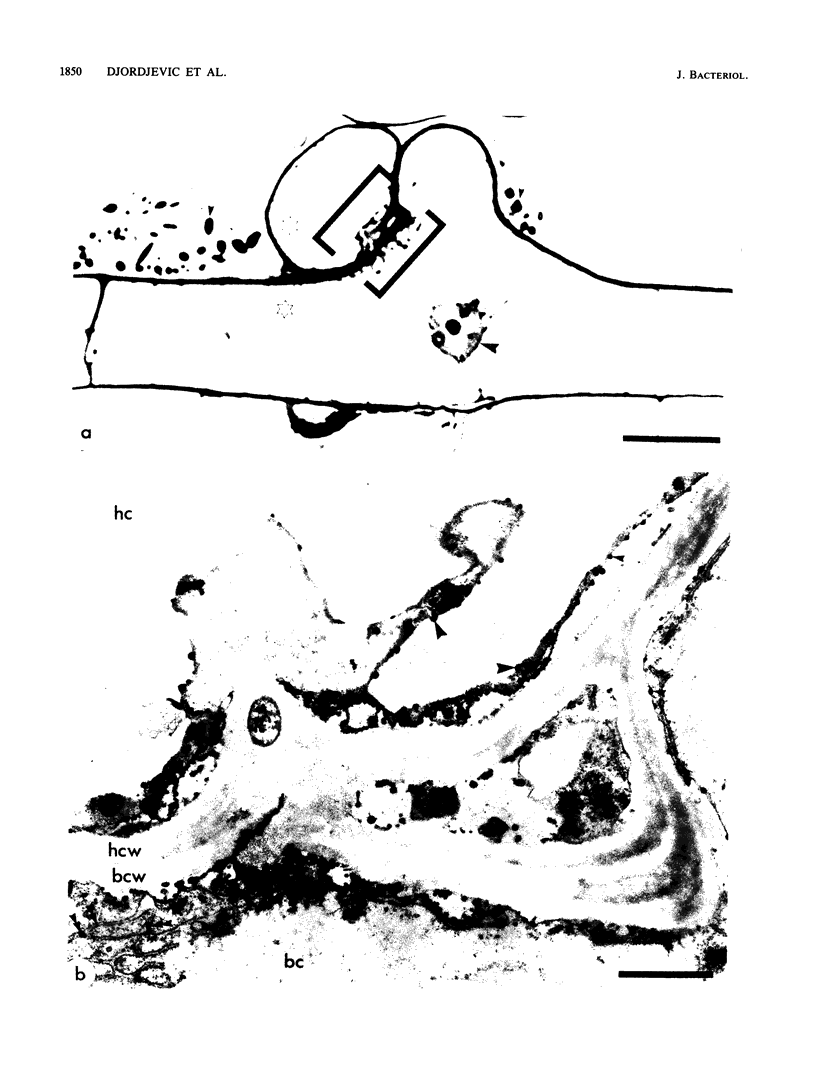
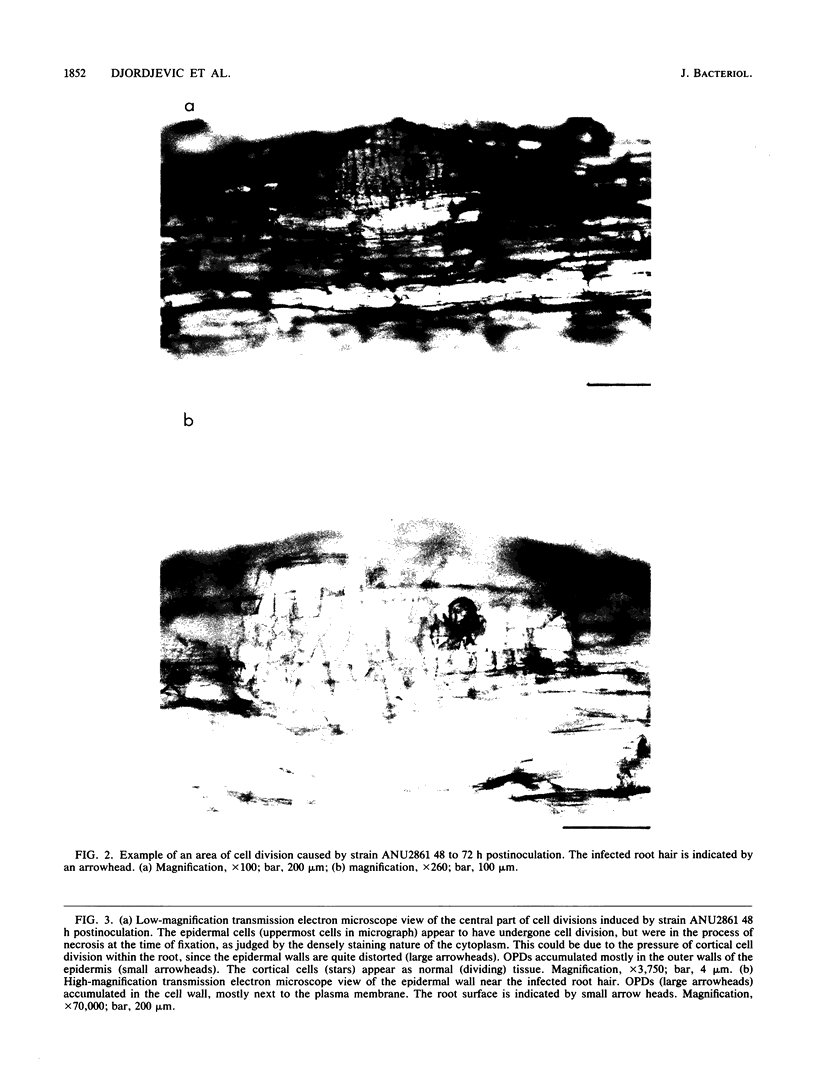
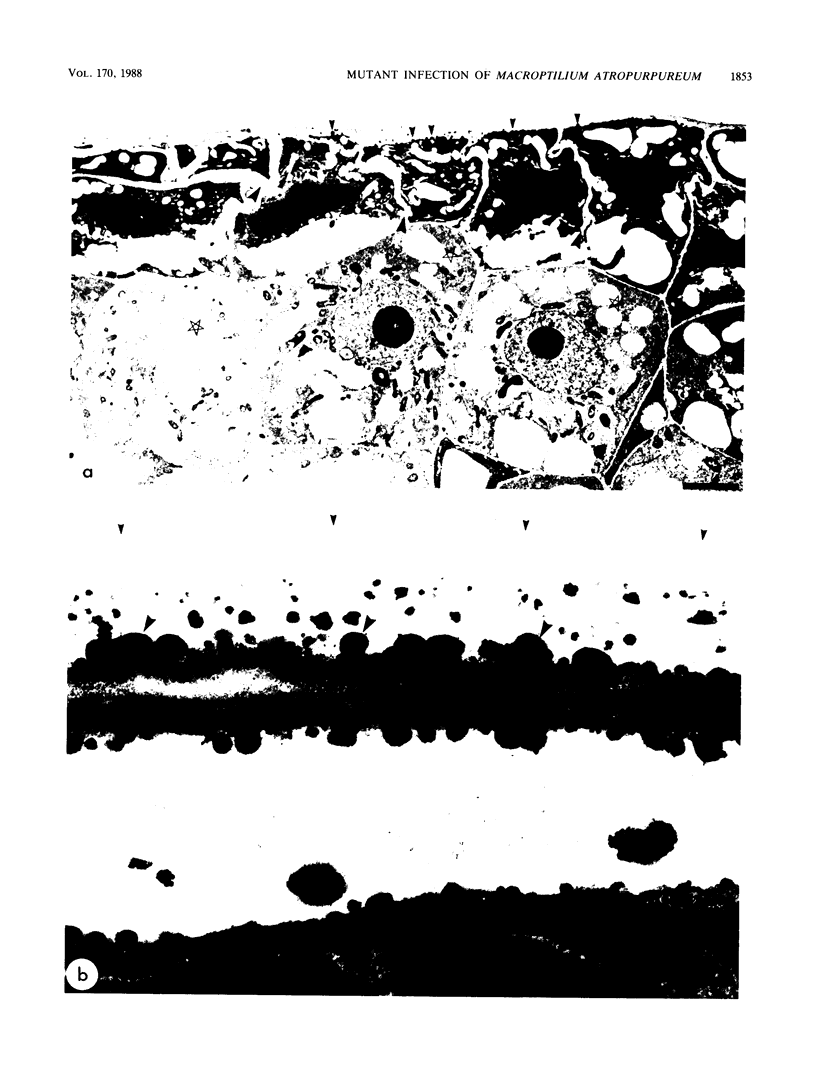
Abstract
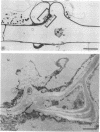
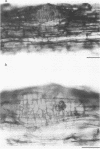
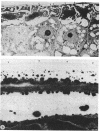
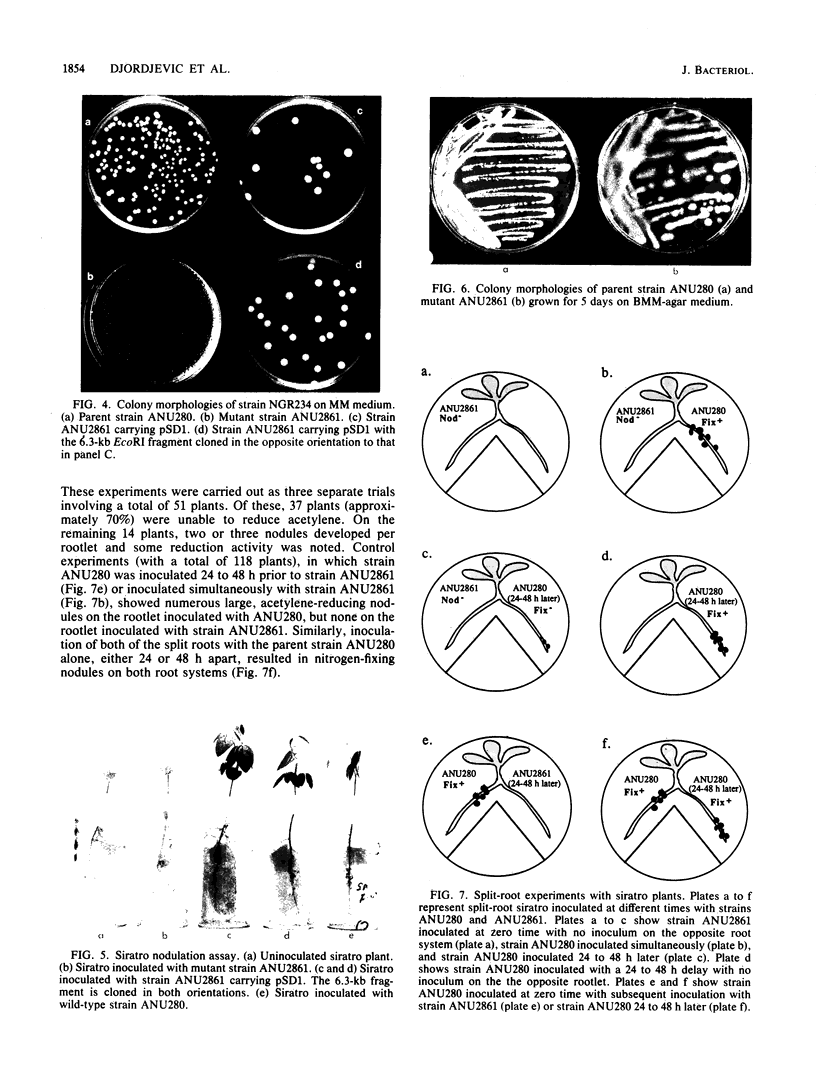
Mutant strain ANU2861, a transposon Tn5 mutant of the fast-growing, broad-host-range Rhizobium strain ANU280 (NGR234 Smr Rfr) overproduces polysaccharide, is an ade auxotroph, and induces poorly developed nodules on Leucaena leucocephala and Lablab purpureus (H.C. Chen, M. Batley, J.W. Redmond, and B.G. Rolfe, J. Plant Physiol. 120:331-349, 1985). Strain ANU2861 cannot form nodules on Macroptilium atropurpureum Urb. (siratro) or on Desmodium intortum and D. uncinatum and the nonlegume Parasponia. The parent strain, ANU280, effectively nodulates all these legume species except Parasponia, on which it forms ineffective nodules. Ultrastructural examination of infection sites on the legume siratro showed that mutant strain ANU2861 caused root hair curling (Hac+ phenotype), some cortical cell division (Noi+), but no infection threads (Inf-). Localized cellular responses, known to occur in phytopathological interactions, were observed in electron micrographs of the epidermal tissue at or near the infection zone after inoculation with strain ANU2861 but not the wild-type parental strain. These include (i) the rapid (within 20 h) accumulation of osmiophilic droplets attached to membranes at potential sites of strain ANU2861 penetration and (after 48 h) in the epidermal cells in the immediate region of the curled root hairs, and (ii) localized cell death of the epidermal cells. In addition, strain ANU2861 can initiate a systemic response in split-root siratro plants which prevents the successful nodulation of strain ANU280. A 6.3-kilobase fragment of wild-type genomic DNA, which includes the site of Tn5 insertion in strain ANU2861, was cloned and introduced to strain ANU2861. All the phenotypic defects of the mutant strain were corrected by the introduction of this DNA fragment. This indicates that the original Tn5 insertion is responsible for the phenotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cen Y., Bender G. L., Trinick M. J., Morrison N. A., Scott K. F., Gresshoff P. M., Shine J., Rolfe B. G. Transposon mutagenesis in rhizobia which can nodulate both legumes and the nonlegume parasponia. Appl Environ Microbiol. 1982 Jan;43(1):233–236. doi: 10.1128/aem.43.1.233-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty A. K., Zurkowski W., Shine J., Rolfe B. G. Symbiotic nitrogen fixation: molecular cloning of Rhizobium genes involved in exopolysaccharide synthesis and effective nodulation. J Mol Appl Genet. 1982;1(6):585–596. [PubMed] [Google Scholar]
- Coplin D. L., Frederick R. D., Majerczak D. R., Haas E. S. Molecular cloning of virulence genes from Erwinia stewartii. J Bacteriol. 1986 Nov;168(2):619–623. doi: 10.1128/jb.168.2.619-623.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic S. P., Chen H., Batley M., Redmond J. W., Rolfe B. G. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J Bacteriol. 1987 Jan;169(1):53–60. doi: 10.1128/jb.169.1.53-60.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylan T., Ielpi L., Stanfield S., Kashyap L., Douglas C., Yanofsky M., Nester E., Helinski D. R., Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett W. F., Osman S. F., Fishman M. L., Siebles T. S. Alginate production by plant-pathogenic pseudomonads. Appl Environ Microbiol. 1986 Sep;52(3):466–473. doi: 10.1128/aem.52.3.466-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Geremia R. A., Cavaignac S., Zorreguieta A., Toro N., Olivares J., Ugalde R. A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form beta-(1----2) glucan. J Bacteriol. 1987 Feb;169(2):880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren P. B., Peet R. C., Panopoulos N. J. Gene cluster of Pseudomonas syringae pv. "phaseolicola" controls pathogenicity of bean plants and hypersensitivity of nonhost plants. J Bacteriol. 1986 Nov;168(2):512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Stacey G., Douglas C. J., Nester E. W. Role for 2-linked-beta-D-glucan in the virulence of Agrobacterium tumefaciens. J Bacteriol. 1985 Oct;164(1):102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Huang S. Z., Rolfe B. G., Djordjevic M. A. Split-Root Assays Using Trifolium subterraneum Show that Rhizobium Infection Induces a Systemic Response That Can Inhibit Nodulation of Another Invasive Rhizobium Strain. Appl Environ Microbiol. 1987 Jul;53(7):1611–1619. doi: 10.1128/aem.53.7.1611-1619.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L. Surface components involved in bacterial pathogen-plant host recognition. J Cell Sci Suppl. 1985;2:301–316. doi: 10.1242/jcs.1985.supplement_2.16. [DOI] [PubMed] [Google Scholar]
- Vance C. P. Rhizobium infection and nodulation: a beneficial plant disease? Annu Rev Microbiol. 1983;37:399–424. doi: 10.1146/annurev.mi.37.100183.002151. [DOI] [PubMed] [Google Scholar]
- Vandenbosch K. A., Noel K. D., Kaneko Y., Newcomb E. H. Nodule initiation elicited by noninfective mutants of Rhizobium phaseoli. J Bacteriol. 1985 Jun;162(3):950–959. doi: 10.1128/jb.162.3.950-959.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. J., Anjaiah V., Nambiar P. T., Ausubel F. M. Isolation and characterization of symbiotic mutants of bradyrhizobium sp. (Arachis) strain NC92: mutants with host-specific defects in nodulation and nitrogen fixation. J Bacteriol. 1987 May;169(5):2177–2186. doi: 10.1128/jb.169.5.2177-2186.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]