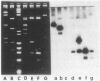
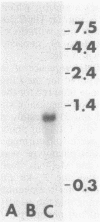
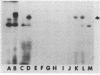
Abstract
Escherichia coli lit(Con) mutations cause a severe inhibition of gene expression late in infection by bacteriophage T4 owing to the overproduction of one, and possibly two, proteins (C. Kao, E. Gumbs, and L. Snyder, J. Bacteriol. 169:1232-1238, 1987). One or both of these proteins interact, either directly or indirectly, with a short sequence about one-quarter of the way into the major capsid protein gene of T4, and the inhibition occurs when this late gene of the virus is expressed. In this report we show that lit(Con) mutations are up-promoter mutations in the cryptic DNA element e14 and that only one of the proteins, gplit, of about 34 kilodaltons, is required for the inhibition. We have sequenced the lit gene and the surrounding regions. From the sequence, and from cell fractionation studies, we conclude that gplit is an inner membrane protein. Since the assembly of T4 heads is thought to occur on the inner face of the inner membrane, we propose that gplit interferes with a normal regulation which coordinates the synthesis of proteins and the assembly of T4 heads.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Benzer S. FINE STRUCTURE OF A GENETIC REGION IN BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):344–354. doi: 10.1073/pnas.41.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H., Greener A., Hill C. W. Excision and reintegration of the Escherichia coli K-12 chromosomal element e14. J Bacteriol. 1985 Mar;161(3):1112–1117. doi: 10.1128/jb.161.3.1112-1117.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Rolfe B. G. Evidence for a dual control of the initiation of host-cell lysis caused by phage lambda. Mol Gen Genet. 1975 Aug 5;139(1):1–8. doi: 10.1007/BF00267990. [DOI] [PubMed] [Google Scholar]
- Champness W. C., Snyder L. Bacteriophage T4 gol site: sequence analysis and effects of the site on plasmid transformation. J Virol. 1984 May;50(2):555–562. doi: 10.1128/jvi.50.2.555-562.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champness W. C., Snyder L. The gol site: a cis-acting bacteriophage T4 regulatory region that can affect expression of all the T4 late genes. J Mol Biol. 1982 Mar 15;155(4):395–407. doi: 10.1016/0022-2836(82)90478-8. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley W., Sirotkin K., Green R., Synder L. A new gene of Escherichia coli K-12 whose product participates in T4 bacteriophage late gene expression: interaction of lit with the T4-induced polynucleotide 5'-kinase 3'-phosphatase. J Bacteriol. 1979 Oct;140(1):83–91. doi: 10.1128/jb.140.1.83-91.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Origin of transfer of IncF plasmids and nucleotide sequences of the type II oriT, traM, and traY alleles from ColB4-K98 and the type IV traY allele from R100-1. J Bacteriol. 1986 Oct;168(1):132–139. doi: 10.1128/jb.168.1.132-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener A., Hill C. W. Identification of a novel genetic element in Escherichia coli K-12. J Bacteriol. 1980 Oct;144(1):312–321. doi: 10.1128/jb.144.1.312-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C., Gumbs E., Snyder L. Cloning and characterization of the Escherichia coli lit gene, which blocks bacteriophage T4 late gene expression. J Bacteriol. 1987 Mar;169(3):1232–1238. doi: 10.1128/jb.169.3.1232-1238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin L. S., Kim Y. J., Meyer R. J. The 20 bp, directly repeated DNA sequence of broad host range plasmid R1162 exerts incompatibility in vivo and inhibits R1162 DNA replication in vitro. Mol Gen Genet. 1987 Jul;208(3):390–397. doi: 10.1007/BF00328129. [DOI] [PubMed] [Google Scholar]
- Maguin E., Brody H., Hill C. W., D'Ari R. SOS-associated division inhibition gene sfiC is part of excisable element e14 in Escherichia coli. J Bacteriol. 1986 Oct;168(1):464–466. doi: 10.1128/jb.168.1.464-466.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Malamy M. H. Regulation of the F-factor pif operon: pifO, a site required in cis for autoregulation, titrates the pifC product in trans. J Bacteriol. 1984 Oct;160(1):192–198. doi: 10.1128/jb.160.1.192-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinedling S., Parma D., Gold L. Wild-type bacteriophage T4 is restricted by the lambda rex genes. J Virol. 1987 Dec;61(12):3790–3794. doi: 10.1128/jvi.61.12.3790-3794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart P., Ito M., Stewart C., Conrad S. E. Induction of cellular thymidine kinase occurs at the mRNA level. Mol Cell Biol. 1985 Jun;5(6):1490–1497. doi: 10.1128/mcb.5.6.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toothman P., Herskowitz I. Rex-dependent exclusion of lambdoid phages. I. Prophage requirements for exclusion. Virology. 1980 Apr 15;102(1):133–146. doi: 10.1016/0042-6822(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]