Abstract
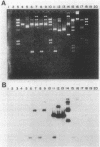
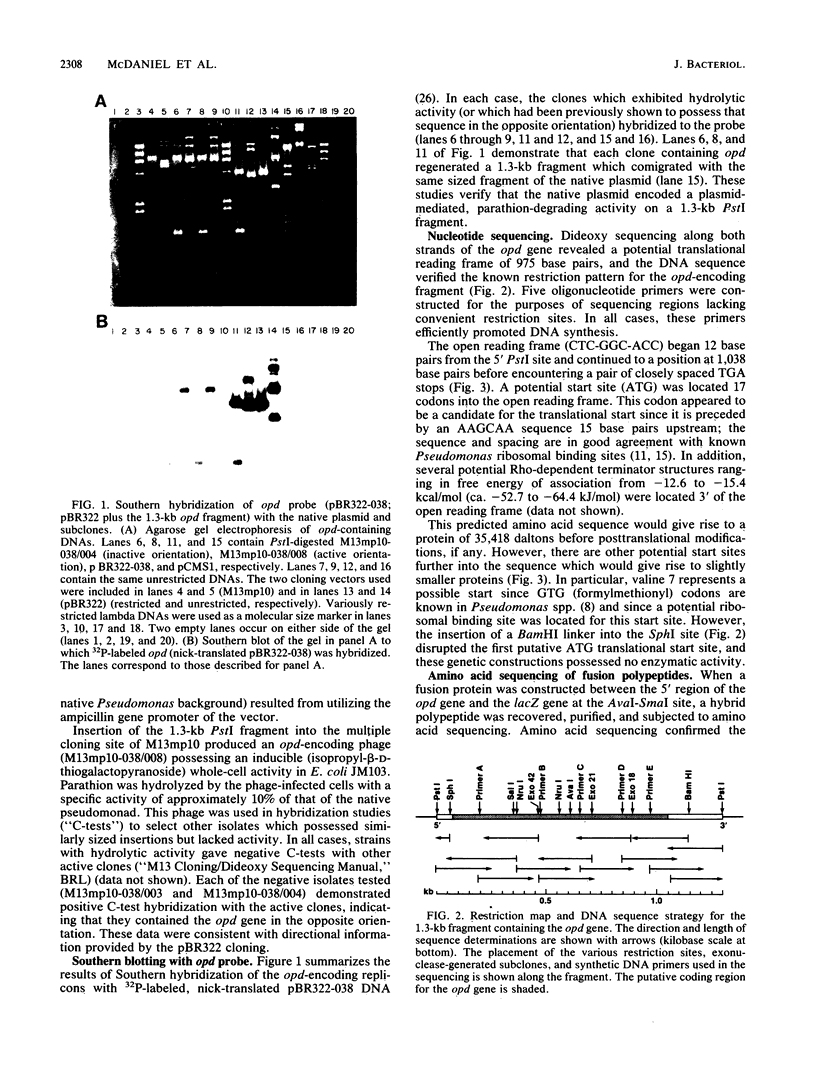
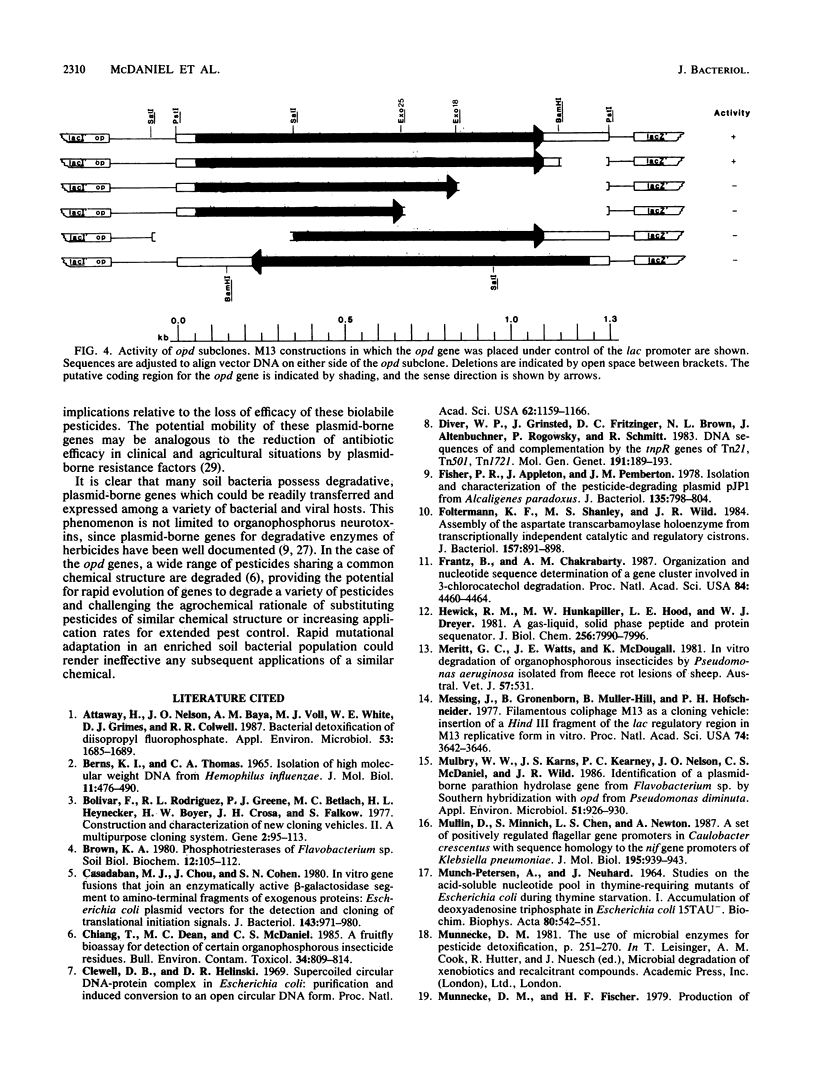
Plasmid pCMS1 was isolated from Pseudomonas diminuta MG, a strain which constitutively hydrolyzes a broad spectrum of organophosphorus compounds. The native plasmid was restricted with PstI, and individual DNA fragments were subcloned into pBR322. A recombinant plasmid transformed into Escherichia coli possessed weak hydrolytic activity, and Southern blotting with the native plasmid DNA verified that the DNA sequence originated from pCMS1. When the cloned 1.3-kilobase fragment was placed behind the lacZ' promoter of M13mp10 and retransformed into E. coli, clear-plaque isolates with correctly sized inserts exhibited isopropyl-beta-D-thiogalactopyranoside-inducible whole-cell activity. Sequence determination of the M13 constructions identified an open reading frame of 975 bases preceded by a putative ribosome-binding site appropriately positioned upstream of the first ATG codon in the open reading frame. An intragenic fusion of the opd gene with the lacZ gene produced a hybrid polypeptide which was purified by beta-galactosidase immunoaffinity chromatography and used to confirm the open reading frame of opd. The gene product, an organophosphorus phosphotriesterase, would have a molecular weight of 35,418 if the presumed start site is correct. Eighty to ninety percent of the enzymatic activity was associated with the pseudomonad membrane fractions. When dissociated by treatment with 0.1% Triton and 1 M NaCl, the enzymatic activity was associated with a molecular weight of approximately 65,000, suggesting that the active enzyme was dimeric.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attaway H., Nelson J. O., Baya A. M., Voll M. J., White W. E., Grimes D. J., Colwell R. R. Bacterial detoxification of diisopropyl fluorophosphate. Appl Environ Microbiol. 1987 Jul;53(7):1685–1689. doi: 10.1128/aem.53.7.1685-1689.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T., Dean M. C., McDaniel C. S. A fruit fly bioassay with phosphotriesterase for detection of certain organophosphorus insecticide residues. Bull Environ Contam Toxicol. 1985 Jun;34(6):809–814. doi: 10.1007/BF01609811. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver W. P., Grinsted J., Fritzinger D. C., Brown N. L., Altenbuchner J., Rogowsky P., Schmitt R. DNA sequences of and complementation by the tnpR genes of Tn21, Tn501 and Tn1721. Mol Gen Genet. 1983;191(2):189–193. doi: 10.1007/BF00334812. [DOI] [PubMed] [Google Scholar]
- Fisher P. R., Appleton J., Pemberton J. M. Isolation and characterization of the pesticide-degrading plasmid pJP1 from Alcaligenes paradoxus. J Bacteriol. 1978 Sep;135(3):798–804. doi: 10.1128/jb.135.3.798-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltermann K. F., Shanley M. S., Wild J. R. Assembly of the aspartate transcarbamoylase holoenzyme from transcriptionally independent catalytic and regulatory cistrons. J Bacteriol. 1984 Mar;157(3):891–898. doi: 10.1128/jb.157.3.891-898.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz B., Chakrabarty A. M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- MUNCH-PETERSEN A., NEUHARD J. STUDIES ON THE ACID-SOLUBLE NUCLEOTIDE POOL IN THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI DURING THYMINE STARVATION. I. ACCUMULATION OF DEOXYADENOSINE TRIPHOSPHATE IN ESCHERICHIA COLI 15 T-A-U-. Biochim Biophys Acta. 1964 Apr 27;80:542–551. doi: 10.1016/0926-6550(64)90298-1. [DOI] [PubMed] [Google Scholar]
- Merritt G. C., Watts J. E., McDougall K. In vitro degradation of organophosphorus insecticides by Pseudomonas aeruginosa isolated from fleece-rot lesions of sheep. Aust Vet J. 1981 Nov;57(11):531–531. doi: 10.1111/j.1751-0813.1981.tb05800.x. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulbry W. W., Karns J. S., Kearney P. C., Nelson J. O., McDaniel C. S., Wild J. R. Identification of a plasmid-borne parathion hydrolase gene from Flavobacterium sp. by southern hybridization with opd from Pseudomonas diminuta. Appl Environ Microbiol. 1986 May;51(5):926–930. doi: 10.1128/aem.51.5.926-930.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin D., Minnich S., Chen L. S., Newton A. A set of positively regulated flagellar gene promoters in Caulobacter crescentus with sequence homology to the nif gene promoters of Klebsiella pneumoniae. J Mol Biol. 1987 Jun 20;195(4):939–943. doi: 10.1016/0022-2836(87)90497-9. [DOI] [PubMed] [Google Scholar]
- Munnecke D. M., Hsieh D. P. Microbial decontamination of parathion and p-nitrophenol in aqueous media. Appl Microbiol. 1974 Aug;28(2):212–217. doi: 10.1128/am.28.2.212-217.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A., Alexander M. Microbial cleavage of various organophosphorus insecticides. Appl Environ Microbiol. 1979 May;37(5):886–891. doi: 10.1128/aem.37.5.886-891.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar C. M., Gibson D. T., Munnecke D. M., Lancaster J. H. Plasmid Involvement in Parathion Hydrolysis by Pseudomonas diminuta. Appl Environ Microbiol. 1982 Jul;44(1):246–249. doi: 10.1128/aem.44.1.246-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethunathan N., Yoshida T. A Flavobacterium sp. that degrades diazinon and parathion. Can J Microbiol. 1973 Jul;19(7):873–875. doi: 10.1139/m73-138. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalker D. M., McBride K. E. Cloning and expression in Escherichia coli of a Klebsiella ozaenae plasmid-borne gene encoding a nitrilase specific for the herbicide bromoxynil. J Bacteriol. 1987 Mar;169(3):955–960. doi: 10.1128/jb.169.3.955-960.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Maratea D., Young R. Purification of hybrid beta-galactosidase proteins encoded by phi X174 E phi lacZ and Escherichia coli prlA phi lacZ: a general method for the isolation of lacZ fusion polypeptides produced in low amounts. J Mol Appl Genet. 1985;3(1):18–25. [PubMed] [Google Scholar]
- Waid J. S. The possible importance of transfer factors in the bacterial degradation of herbicides in natural ecosystems. Residue Rev. 1972;44:65–71. doi: 10.1007/978-1-4615-8491-9_4. [DOI] [PubMed] [Google Scholar]