Abstract
Background
Intravenous salbutamol (albuterol) reduces lung water in patients with the acute respiratory distress syndrome (ARDS). Experimental data show that it also reduces pulmonary neutrophil accumulation or activation and inflammation in ARDS.
Aim
To investigate the effects of salbutamol on neutrophil function.
Methods
The in vitro effects of salbutamol on neutrophil function were determined. Blood and bronchoalveolar lavage (BAL) fluid were collected from 35 patients with acute lung injury (ALI)/ARDS, 14 patients at risk from ARDS and 7 ventilated controls at baseline and after 4 days' treatment with placebo or salbutamol (ALI/ARDS group). Alveolar–capillary permeability was measured in vivo by thermodilution (PiCCO). Neutrophil activation, adhesion molecule expression and inflammatory cytokines were measured.
Results
In vitro, physiological concentrations of salbutamol had no effect on neutrophil chemotaxis, viability or apoptosis. Patients with ALI/ARDS showed increased neutrophil activation and adhesion molecule expression compared with at risk‐patients and ventilated controls. There were associations between alveolar–capillary permeability and BAL myeloperoxidase (r = 0.4, p = 0.038) and BAL interleukin 8 (r = 0.38, p = 0.033). In patients with ALI/ARDS, salbutamol increased numbers of circulating neutrophils but had no effect on alveolar neutrophils.
Conclusion
At the onset of ALI/ARDS, there is increased neutrophil recruitment and activation. Physiological concentrations of salbutamol did not alter neutrophil chemotaxis, viability or apoptosis in vitro. In vivo, salbutamol increased circulating neutrophils, but had no effect on alveolar neutrophils or on neutrophil activation. These data suggest that the beneficial effects of salbutamol in reducing lung water are unrelated to modulation of neutrophil‐dependent inflammatory pathways.
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are major causes of respiratory failure in the critically ill patient.1 Although controversy still exists regarding the role of neutrophils in all causes of ALI,2 a recent systematic review of laboratory and clinical studies concluded that neutrophils played a central part in most cases.3 Pathophysiologically, ARDS is characterised by intense inflammation in the alveolar space, with a predominance of neutrophils. Analysis of bronchoalveolar lavage (BAL) fluid from patients with ARDS has shown increased numbers of activated neutrophils in the early stages of ARDS.4,5 The number of neutrophils in BAL fluid relates to the severity of lung injury,6 and the persistence of neutrophils is associated with increased mortality.5 A study examining BAL fluid from patients with ARDS showed a positive correlation between neutrophil myeloperoxidase (MPO) and oxidatively modified amino acids, suggesting an association between pulmonary neutrophil activation and oxidative protein damage.7 Animal studies have shown that neutrophil‐mediated lung injury reduces alveolar fluid clearance, preventing the resolution of non‐cardiogenic pulmonary oedema.8 Neutrophil elastase inhibitors in animal models limit the degree of lung injury caused by ischaemia reperfusion9 and lipopolysaccharide (LPS),10 although a recent multicentre clinical trial of the elastase inhibitor Sivelestat failed to improve outcome in a heterogeneous group of patients with ARDS.11
β2‐agonists have several inhibitory effects on neutrophil function.12 In animal models of acute lung injury, β2‐agonists reduce pulmonary neutrophil sequestration.13,14 In vitro β2‐agonists reduce the production of oxygen free radicals from neutrophils and other inflammatory cells15,16 and reduce inflammatory cytokine production.17 In humans, inhaled salmeterol (long‐acting β2‐agonist) inhibited LPS‐induced neutrophil influx, degranulation and tumour necrosis factor (TNF)α release.18 These experimental findings suggest that treatment with a β2‐agonist could have a favourable effect in ARDS by reducing neutrophilic inflammation.
We recently conducted a randomised controlled trial in humans with ARDS (β‐agonist lung injury trial (BALTI‐1) study), and showed that intravenous salbutamol (albuterol) markedly reduced extravascular lung water probably through up regulation of alveolar fluid clearance.19 However, the reduction in lung water was not seen until 48 h after the initiation of treatment, suggesting some effects in addition to alveolar fluid clearance. The objective of this study was to investigate whether salbutamol modulates neutrophil function and neutrophilic inflammation at physiologically relevant doses in patients with ALI. The second objective was to investigate the relationship between neutrophilic inflammation and alveolar capillary permeability in patients with ARDS.
Materials and methods
In vitro studies
Under‐agarose chemotaxis, cell viability and apoptosis
Neutrophils from non‐smoking, healthy controls were purified from peripheral blood by discontinuous Percoll density gradients.20 The effect of salbutamol on the chemotactic activity of purified neutrophils was measured using the under‐agarose method.21 The freshly harvested neutrophils were resuspended at 5×107 cells/ml in RPMI (Sigma, UK) 1640 culture medium or 10−5–10−10 M salbutamol in RPMI (with or without propranolol 10−4 M) before seeding in 10‐μl aliquots in the central wells of a freshly prepared agarose plate. In all, 10 μl of RPMI was placed in each of the inner wells and 10 μl of chemoattractant (10−7 N‐formyl‐l‐leucin‐methionyl‐l‐phenylalanine (FMLP, Sigma, UK) in RPMI) in each of the outer wells. Negative controls contained RPMI in the inner and outer wells. The plates were incubated at 37°C (5% CO2) for 2 h and then flooded with methanol for overnight fixation of the cells. The gel was carefully removed and the plates washed under slow running tap water. The plates were then stained with Gram stain and left to dry. For each well, the chemotactic and chemokinetic responses were read using an eyepiece graticule. The overall response to the chemoattractant (chemotactic differential) was calculated by subtracting the chemokinetic response from the chemotactic response.
Purified neutrophils, suspended in RPMI at a concentration of 1×106 ml, were incubated with 10−5 and 10−7 M salbutamol or RPMI control for 2 h at 37°C in humidified 5% CO2. Cell proliferation was assessed by adding 20 μl of CellTiter 96 AQueous one solution (Promega, Southampton, UK) to 100 μl of cell suspension in a 96‐well culture plate (Nunc). The reaction was allowed to proceed for 2 h at 37°C in 5% CO2. The cell titre solution contains a tetrazolium compound that is metabolised by healthy cells to a formazan product, the absorbance of which was read at 495 nm on an MRX‐II 96‐well plate reader (Dynex Technologies, Sussex, UK).
Purified neutrophils, suspended in RPMI with 10% heat‐inactivated fetal calf serum (Life Technologies) and 100 units/ml penicillin and 100 mg/ml streptomycin (Sigma, Poole, UK) at a concentration of 1×106 ml, were incubated with 10−5 and 10−7 M salbutamol or RPMI control for 18 h at 37°C in humidified 5% CO2. Preliminary experiments showed that 18 h was the optimal time point for assessing apoptosis. The number of normal and apoptotic neutrophils were determined by morphological patterns. After centrifugation, samples were stained using a commercial May–Grunwald Giemsa stain (Diff‐Quick, Baxter Healthcare Product, Deerfield, Illinois, USA). The percentage apoptotic cells compared with total number of cells were calculated. The morphological results were confirmed using flow cytometry and the annexin V/propidium iodine apoptosis kit (Dako, Carpinteria, California, USA). The percentages of live (annexin and propidium iodine negative) and early apoptotic (annexin positive, prodipium iodine negative) or late apoptotic/dead (annexin positive, prodipium iodine positive) cells were calculated.
Clinical study
Mechanically ventilated adult patients enrolled in the BALTI‐1 study were eligible for inclusion.19 This study recruited patients within 48 h of the onset of ALI and ARDS and randomised them to 7 days of treatment with intravenous salbutamol (15 μg/kg/h). ALI was defined according to the American European Consensus Conference definition22 as the acute onset of respiratory failure with a arterial oxygen tension:fractional oxygen tension (PaO2:FiO2) ratio of <300 mm Hg and bilateral infiltrates on the chest radiograph in the absence of clinical evidence of left atrial hypertension. ARDS was considered present when the PaO2:FiO2 ratio was <200 mm Hg. The exclusion criteria were as follows: age <18 years; participation in other intervention trials; severe obstructive airway disease requiring nebulised or intravenous β2‐agonist; treatment with β‐blockers within 48 h; neutrophil count <0.3×109 l; brain stem death; treatment withdrawal within 24 h; immunosuppression (steroids >20 mg/day, chemotherapy or other immunosuppressive agents within 2 weeks); lobectomy/pneumonectomy; burns >40% body surface area; assent declined from the next of kin.
Patients with identifiable risk factors for ALI/ARDS, but who, at the time of recruitment had not met the criteria for ALI/ARDS, were included as an at risk‐population. Non‐smoking, age‐matched ventilated patients undergoing elective surgery were recruited as controls.
Biological sample collection and processing
BAL using 150 ml cold saline was performed immediately after recruitment in at‐risk patients and ventilated controls and at baseline and on day 4 in patients with ALI/ARDS. BAL fluid was kept on ice until transferred to the laboratory for processing. Blood was simultaneously collected in lithium heparin tubes (Becton‐Dickinson, Plymouth, UK) and placed immediately on ice until processed.
The BAL fluid was filtered through coarse surgical gauze to remove mucus and other debris. The fluid was then centrifuged at 500 g for 5 min in a centrifuge prechilled to 4°C. The supernatant was removed and immediately frozen to −80°C and stored for subsequent analysis. The cell pellet was resuspended in 10 ml phosphate‐buffered saline supplemented with 1% human serum albumin. Total cell count was determined using a haemocytometer. Cell viability was measured by the ability of live cells to exclude Trypan Blue. Cell purity was measured on a cytospin preparation stained with DiffQuick (Baxter, Berkshire, UK) as described previously .23
Patient characteristics
Patient demographic characteristics were recorded at baseline. The acute physiology and chronic health evaluation II (APACMEII) and simplified acute physiology score II (SAPSII) scores, and predicted intensive care unit mortality were recorded as global markers of disease severity.24 The Murray Lung Injury Score and PaO2:FiO2 ratio were collected as markers of the severity of lung injury.
Alveolar–capillary permeability
The single indicator transpulmonary thermodilution system (PiCCO; Pulsion Medical Systems, Munich, Germany) was used to calculate an in vivo alveolar–capillary permeability index. The permeability index was derived from the ratio of extravascular lung water to pulmonary blood volume. Previous studies have shown that this index can separate cardiogenic (low permeability) and inflammatory (high permeability) causes of pulmonary oedema.25
Flow cytometry analysis
Flow cytometry was performed using the whole‐blood technique.26 Aliquots of 100 μl of whole blood or BAL cells (suspended in 1% human serum albumin (Sigma Chemicals) at a final concentration of 1×106/ml) were fixed by adding 100 μl of 1% paraformaldehyde for 15 min. Immunofluorescent staining was performed by adding fluorescin isothiocyante isomer‐conjugated monoclonal antibodies directed against the following: CD11B (IgG1, Dako, Ely, UK); CD18 (IgG1, Dako); CD49 (IgG1, Serotec, Oxford, UK), CD64 (IgG1, Serotec) and l‐selectin, IgG1, (Becton‐Dickinson). Stimulated neutrophil l‐selectin expression was determined by incubating whole blood with FMLP (10−6 M) for 15 min before fixation. Controls were included using isotype‐matched, irrelevant antibodies to human IgG1 (Dako). After incubation with the appropriate monoclonal antibody for 30 min at room temperature (protected from light), 1.5 ml of FACS brand lysing solution (Becton‐Dickinson) was added to each tube for 5 min. The cells were then washed twice with 1.5 ml of wash buffer (500 ml phosphate‐buffered saline, 0.1 g sodium azide (Sigma Chemicals), 5 g bovine serum albumin (Sigma)). After the second wash, the cell pellet was resuspended in 500 μl of 1% paraformaldehyde. Samples were analysed on a Becton‐Dickinson 440 flow cytometer. The neutrophil cell populations were identified and gated from the forward and side light scatter. The median intensity of fluorescence for cells labelled with specific antibody was determined relative to the median intensity of fluorescence for cells labelled with the non‐specific isotype control antibody.
Tumor necrosis factor α, interleukin 8, MPO and salbutamol assays
Tumor necrosis factor α (TNFα) and interleukin (IL)8 levels in BAL fluid were measured using a commercially available ELISA (R&D systems, Abingdon, UK). BAL MPO was measured using the chromogenic substrate assay as described previously.23 Salbutamol levels were measured using a commercially available ELISA (Biox diagnostics, Jemelle, Belgium).
Statistical analysis
The study was powered to detect a 60% reduction in alveolar neutrophil sequestration based on recent data showing that β2‐agonists reduce pulmonary neutrophil sequestration in human volunteers exposed to LPS by 60%.18 We calculated that nine patients would need to be recruited in each arm to detect this difference with 80% power at a significance level of 0.05. Differences between groups were examined by analysis of variance or the Kruskal–Wallis test. Where significant differences were identified, Tukey's test or the Mann–Whitney U test was used to further examine the differences. Repeated measures of neutrophil counts were non‐parametric and analysed by Friedman's repeated measures test. Linear associations were tested using Pearsons correlation test. Data are expressed as mean (standard deviation) unless otherwise stated; p <0.05 was considered significant.
Results
In vitro studies
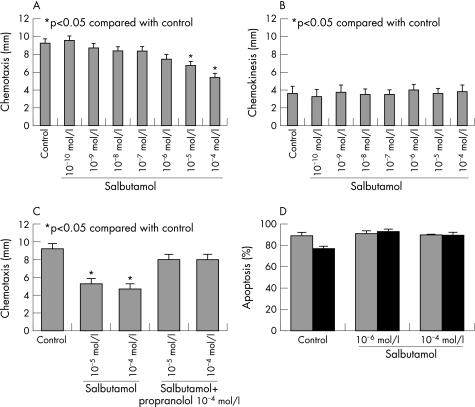
Neutrophil migration towards the chemotractant stimulus FMLP was greater than that towards the RPMI control (11.6 mm (1.2) versus 3.6 mm (0.2), p<0.001). Salbutamol at 10−4 and 10−5 M significantly reduced neutrophil chemotaxis (fig 1A). There was no effect on random neutrophil chemokinesis (fig 1B). The effect of salbutamol on neutrophil chemotaxis was abolished by propranolol (fig 1C). Salbutamol 10−4 to 10−6 M had no effect on cellular viability (data not shown) or neutrophil apoptosis (fig 1D).
Figure 1 (A) Salbutamol 10−5 M and 10−4 M significantly reduced neutrophil chemotaxis towards the chemotractant stimulus FMLP. (B) No effect was seen on neutrophil chemokineses. (C) The effect of salbutamol on neutrophil chemotaxis was blocked by incubation with 10−4 M propranolol. (D) Salbutamol (10−6 and 10−4 M) had no effect on the rate of apoptosis at 18 h (apoptosis determined by morphology (grey bars) and flow cytometry (black bars). Data shown are mean (standard error) from six experiments. *p<0.05 compared with control.
Clinical studies
Patient baseline demographics
Forty patients with ALI/ARDS were initially recruited, of whom 35 had BAL performed at baseline and were included in this study. Fourteen patients with risk factors for ARDS but who had not developed ARDS at the time of BAL formed the at risk group. Some of the data from the at risk group (BAL MPO levels and neutrophil counts) have been published previously.23 Seven ventilated controls were recruited. Table 1 shows the baseline data on severity of illness and aetiology of lung injury for the patients with ARDS and those at risk.
Table 1 Baseline demographics for patients at risk from acute respiratory distress syndrome and patients with established acute respiratory distress syndrome.
| ARDS (n = 35) | At risk (n = 19) | p Value | 95% CI | |
|---|---|---|---|---|
| P:F ratio mm Hg (kPa) | 106 (92) (33.5 (12.1)) | 155 (92) (14.9 (5.8)) | <0.001 | 95–185 (12.5 to 24.4) |
| Lung injury score | 2.9 (0.6) | 1.2 (0.3) | 0.001 | 1.3 to 1.9 |
| SOFA score | 13.4 (3.7) | 6.7 (4.3) | 0.001 | 4.1 to 9.2 |
| APACHE II | 23.4 (6.9) | 21.7 (8.9) | 0.312 | −2.1 to 6.5 |
| SAPS II | 52.0 (15.7) | 54.8 (12.8) | 0.550 | −12.0 to 6.4 |
| Direct lung injury | 13 (37%) | 6 (32%) | 0.771 | |
| Indirect lung injury | 22 (63%) | 13 (68%) |
APACHE, acute physiology and chronic health evaluation; ARDS, acute respiratory distress syndrome; SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment.
Neutrophilic inflammation is present in the alveolar space in patients with ARDS
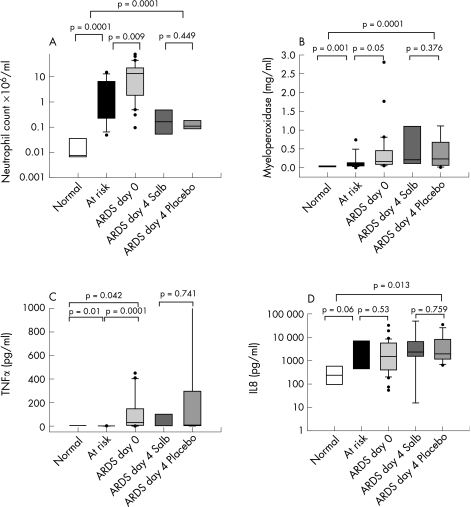
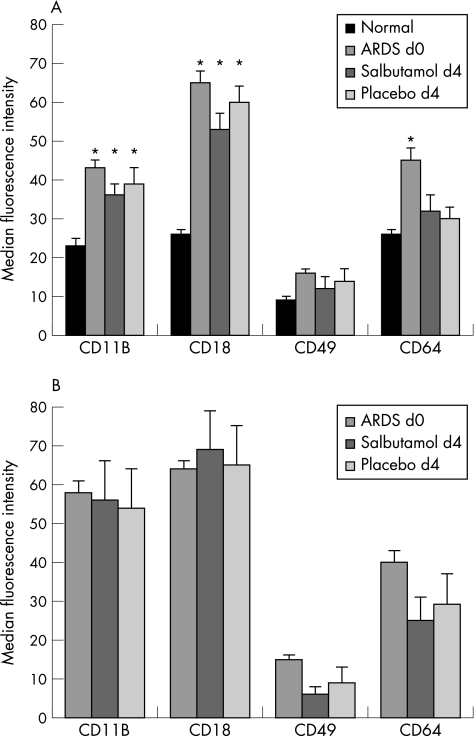
Evidence of increased accumulation of neutrophils and neutrophilic inflammation was found in the BAL fluid of patients with ARDS. Total neutrophil count, MPO, TNFα and IL8 levels were raised in patients with ARDS compared with patients at risk from ARDS and normal ventilated controls (fig 2 (A–D)). We found significant, albeit weak, linear associations between the PiCCO alveolar–capillary permeability index and MPO (r = 0.4, p = 0.038) and IL8 (r = 0.37, p = 0.033) levels. Compared with normal controls, there was evidence of increased adhesion molecule expression CD11B, CD18, CD49, reduced l‐selectin expression and increased neutrophil activation (CD64) on circulating and alveolar neutrophils (fig 3).
Figure 2 (A) Bronchoalveolar lavage neutrophil count, (B) myeloperoxidase, (C) tumour necrosis factor α (TNFα) and (D) interleukin (IL)8 in normals, patients at risk from acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) and patients with ALI/ARDS at baseline and after 4 days of treatment with intravenous salbutamol (salb).
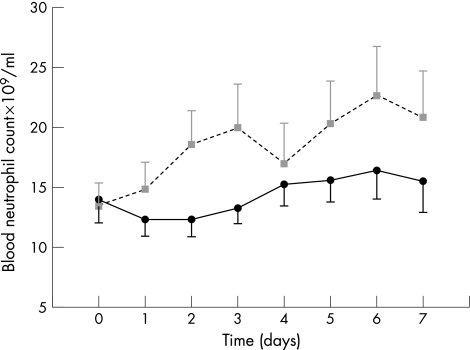
Figure 3 Treatment with intravenous salbutamol significantly increased circulating neutrophil count (p = 0.004). Data are shown as mean (SE) for salbutamol (squares/grey dotted line) and placebo (circles/solid line).
In vivo effects of salbutamol
From the initial group of patients randomised to salbutamol or placebo, follow‐up samples of blood and BAL fluid were collected from 22 patients at day 4 (9 randomised to salbutamol and 13 to placebo). Salbutamol was detected by ELISA in the plasma of the treatment group at a concentration equivalent to 10−6 M (range 250–495 ng/ml). The ELISA did not perform reproducibly in the BAL matrix.
Circulating neutrophils
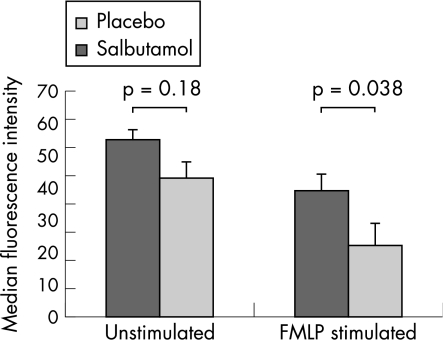
Treatment with intravenous salbutamol significantly increased the number of circulating neutrophils (fig 4). There was a trend towards reduced l‐selectin expression on unstimulated neutrophils in the salbutamol group (placebo 55 (19) v salbutamol 39 (30), p = 0.18). When neutrophils were stimulated ex vivo with FMLP, neutrophils from salbutamol‐treated patients had significantly lower l‐selectin expression (placebo 43 (15) v salbutamol 22 (21), 95% confidence interval (CI) 1.2 to 39, p = 0.038; fig 5). We found no effect on integrin adhesion molecule expression measured at day 4 (CD11B, CD18 or CD49) or expression of the neutrophil activation marker CD64 (fig 3A).
Figure 4 (A) Circulating and (B) alveolar neutrophil adhesion molecule (CD11b, CD18 and CD49) and activation maker (CD64) expression in normal volunteers and patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) at baseline and after 4 days of treatment with intravenous salbutamol. Data shown are mean (SE).
Figure 5 Effect of systemic treatment with intravenous salbutamol on resting and N‐formyl‐l‐leucin‐methionyl‐l‐phenylalanine (FMLP) stimulated neutrophil l‐selectin expression. Data shown are mean (SE).
Pulmonary neutrophils and alveolar inflammation
In contrast to the plasma compartment, there was no difference in BAL fluid neutrophil count between groups at day 4 (placebo 3.5 (0.6) v salbutamol group 1.6 (1.7), p = 0.4; fig 2A). Adhesion molecule expression was also similar in the two groups and there was no difference in the expression of the neutrophil activation marker CD64 (fig 3B). We found no difference in l‐selectin expression (unstimulated cells: placebo 3 (4) v salbutamol 4 (6), p = 0.753; FMLP stimulated cells: placebo 1.7 (2) v salbutamol 1.8 (3), p = 0.9). We also found no differences in myeloperoxidase (fig 2B), TNFα (fig 2C) or IL8 (fig 2D).
Discussion
In a double‐blind randomised placebo‐controlled trial, we showed that sustained treatment with intravenous salbutamol considerably reduced extravascular lung water in humans with ALI/ARDS.19 In contrast with our initial hypothesis and supporting data from animal studies,27 the response was not evident until 48 h after the initiation of treatment. Our study sought to identify whether salbutamol was having an effect on pulmonary neutrophil accumulation and alveolar inflammation. Our study confirms previous observations that there is increased neutrophil accumulation and inflammation in the alveolar space in people with ALI/ARDS. The novel finding of a positive association between the intensity of neutrophilic infiltration and an in vivo measurement of alveolar–capillary permeability adds additional support to the hypothesis that the neutrophil is a key inflammatory mediator in ARDS. Contrary to in vitro, animal and healthy volunteer studies, we found no evidence that intravenous salbutamol modulated alveolar neutrophil accumulation, activation or markers of alveolar inflammation in humans with ALI/ARDS.
Studies investigating the in vitro effects of β‐agonists on neutrophil chemotaxis have produced conflicting results, probably due to differences in the specific β2‐agonist tested, the dose used and the experimental conditions. Some studies showed a reduction in neutrophil chemotaxis,28,29 whereas others reported a biphasic response with increased neutrophil chemotaxis at low concentrations of β2‐agonist and a reduction in chemotaxis at higher concentrations.30 Our study reports for the first time that the physiological concentration of salbutamol achieved in the plasma after an intravenous infusion of salbutamol (15 μg/kg/h) in patients with ALI/ARDS is 10−6 M. At this concentration, no effect on neutrophil chemotaxis was observed. Supraphysiological concentrations (10−4 and 10−5 M), however, reduced neutrophil chemotaxis through activation of the β‐receptor. Moreover, physiologically relevant concentrations of salbutamol had no effect on neutrophil viability or the rate of spontaneous apoptosis measured by morphology and annexin V/propidium iodine staining.
The mechanisms regulating pulmonary neutrophil sequestration have been well characterised. Pulmonary neutrophil sequestration occurs within minutes of exposure to an inflammatory insult.31,32 The insult causes an increase in neutrophil stiffness and reduction in deformability,33 leading to sequestration into the pulmonary capillaries, followed by emigration into the alveolar space. The process of neutrophil emigration occurs by at least two differentially regulated pathways: CD11/18 adhesion molecule interactions determine the response to Gram‐negative organisms, IL1α and phorbol 12‐myristate 13‐acetate, whereas Gram‐positive organisms, hyperoxia and the complement anaphylatoxins (C5a) seem to induce neutrophil emigration through a CD11/18‐independent pathway.34
Animal models of direct13 and indirect14 lung injury have shown that pretreatment with intravenous β2‐agonists reduces pulmonary neutrophil sequestration by 30%. In normal human volunteers, in a placebo‐controlled trial, treatment with 300 μg inhaled salbutamol prevented platelet‐activating factor‐induced pulmonary sequestration of radiolabelled neutrophils.35 Pretreatment with salmeterol similarly inhibited LPS‐induced neutrophil influx, neutrophil degranulation (myeloperoxidase) and TNFα release in human volunteers.18 Reduced neutrophil–endothelial adhesion, through the down regulation of neutrophil integrin adhesion molecule expression (CD11B/18) seen with β2‐agonists may partly explain this finding.36
In our study, intravenous salbutamol considerably increased circulating neutrophil count but had no apparent effect on alveolar neutrophil counts. The increase in circulating neutrophils induced by β‐agonists is thought to be due to detachment of cells from the marginating neutrophil pools rather than mobilisation of neutrophils from the bone marrow.37 Neutrophil l‐selectin expression may be important in this effect because of a dominant role in the initial slowing, margination and rolling behaviour of neutrophils over the endothelim at the post‐capillary venule in the systemic circulation.38 The shedding of l‐selectin from the neutrophil surface allows neutrophils to break free from the vascular endothelium and return to the circulation. The mobilisation of neutrophils was not, however, associated with any difference in the degree of organ dysfunction between the two groups.19
In contrast to the systemic circulation, where neutrophil sequestration usually occurs at the post‐capillary venule, neutrophil migration in the lung occurs at the pulmonary capillaries, without dependence on l‐selectin‐mediated rolling.39 In our study, we found a trend towards reduced l‐selectin expression on circulating neutrophils in patients treated with salbutamol. When the neutrophils were maximally stimulated with FMLP, neutrophils primed by treatment with salbutamol showed a notably greater reduction in l‐selectin expression than those treated with placebo. This finding is consistent with the observation that isoprotenerol reduces neutrophil l‐selectin expression in human volunteers.40 Enhanced l‐selectin shedding would increase neutrophil mobilisation from the systemic circulation and may explain the finding of increased circulating neutrophils. By contrast, the absence of a measurable effect of salbutamol on integrin adhesion molecule expression relevant to pulmonary neutrophil emigration may explain the observed lack of effect on pulmonary neutrophil sequestration.
There are several other potential explanations for the absence of a measurable effect of salbutamol in vivo on alveolar neutrophil accumulation and inflammation. Firstly, pulmonary neutrophil emigration occurs within minutes of exposure to the toxic insult.31,32 In the previous studies that reported a reduction in pulmonary neutrophil sequestration with β‐agonists, the drug was administered before the inflammatory insult.13,14,18,35 In our study, salbutamol was given many hours after the initial insult, potentially therefore too late to have an effect on pulmonary neutrophil recruitment. Although the study was relatively small, it is similar in size to previous studies,13,14,18,35 and was powered to detect a treatment effect of a magnitude similar to that observed in previous studies. We cannot, however, exclude that a smaller treatment effect would not have been observed with greater numbers, although the clinical relevance of smaller changes is unclear. Further, multiple signalling pathways enhance neutrophil emigration in patients with ARDS, which may differ depending on the aetiology of ARDS. Our study was not powered to investigate the effects of salbutamol on neutrophil inflammation because of different aetiologies, and so cannot exclude an effect in some causes of ARDS. Finally, alveolar neutrophil recruitment was only studied at baseline and 4 days after the initiation of treatment (up to 6 days after the onset of ARDS). In both treatment and placebo groups, neutrophil counts were markedly lower on day 4 than at the point of initial recruitment. Therefore, we cannot exclude that salbutamol may have had an effect earlier in the course of the disease, which by virtue of the timing of alveolar sampling, may have been missed.
Conclusion
At the onset of ALI/ARDS, there is increased pulmonary neutrophil recruitment and activation in addition to positive correlations between IL8, myeloperoxidase and alveolar–capillary permeability, suggesting an association between neutrophil activation and the development of lung injury. In vitro, physiological concentrations of salbutamol failed to show an effect on neutrophil chemotaxis, viability or apoptosis. Treating patients with ALI/ARDS with intravenous salbutamol increased the number of circulating neutrophils, but had no effect on alveolar neutrophil numbers or on neutrophil activation or alveolar inflammation. The beneficial effects of salbutamol in reducing extravascular lung water seem to be unrelated to the modulation of neutrophilic inflammatory pathways.
Abbreviations
ALI - acute lung injury
ARDS - acute respiratory distress syndrome
BAL - bronchoalveolar lavage
FMLP - N‐formyl‐l‐leucin‐methionyl‐l‐phenylalanine
LPS - lipopolysaccharide
TNF - tumour necrosis factor
Footnotes
Funding: This study was supported by a grant from the West Midlands Intensive Care Society, Queen Elizabeth Hospital, Birmingham, UK.
Competing interests: GDP, DFMcA and DRT have received payment to attend scientific meetings and have given talks for pharmaceutical companies that manufacture β‐ agonists (Astra Zeneca, GlaxoSmithKline).
Ethical approval: This study was approved by the East Birmingham Research Ethics Committee (Reference SJR/LMH/0522).
References
- 1.Rubenfeld G D, Caldwell E, Peabody E.et al Incidence and outcomes of acute lung injury. N Engl J Med 20053531685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Laufe M D, Simon R H, Flint A.et al Adult respiratory distress syndrome in neutropenic patients. Am J Med 1986801022–1026. [DOI] [PubMed] [Google Scholar]
- 3.Abraham E. Neutrophils and acute lung injury. Crit Care Med 200331S195–S199. [DOI] [PubMed] [Google Scholar]
- 4.Chollet‐Martin S. Polymorphonuclear neutrophil activation during the acute respiratory distress syndrome. Intens Care Med 2000261575–1577. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg K P, Milberg J A, Martin T R.et al Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 1994150113–122. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair D G, Braude S, Haslam P L.et al Pulmonary endothelial permeability in patients with severe lung injury. Clinical correlates and natural history. Chest 1994106535–539. [DOI] [PubMed] [Google Scholar]
- 7.Lamb N J, Gutteridge J M, Baker C.et al Oxidative damage to proteins of bronchoalveolar lavage fluid in patients with acute respiratory distress syndrome: evidence for neutrophil‐mediated hydroxylation, nitration, and chlorination [see comments]. Crit Care Med 1999271738–1744. [DOI] [PubMed] [Google Scholar]
- 8.Pittet J F, Lu L N, Morris D G.et al Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol 20011666301–6310. [DOI] [PubMed] [Google Scholar]
- 9.Carden D, Xiao F, Moak C.et al Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am J Physiol 1998275H385–H392. [DOI] [PubMed] [Google Scholar]
- 10.Sakamaki F, Ishizaka A, Urano T.et al Effect of a specific neutrophil elastase inhibitor, ONO‐5046, on endotoxin‐induced acute lung injury. Am J Respir Crit Care Med 1996153391–397. [DOI] [PubMed] [Google Scholar]
- 11.Zeiher B G, Artigas A, Vincent J L.et al Neutrophil elastase inhibition in acute lung injury: results of the STRIVE Study. Crit Care Med 2004321695–1702. [DOI] [PubMed] [Google Scholar]
- 12.Perkins G D, McAuley D F, Richter A.et al Bench‐to‐bedside review: β2‐agonists and the acute respiratory distress syndrome. Crit Care 2004825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhingra V K, Uusaro A, Holmes C L.et al Attenuation of lung inflammation by adrenergic agonists in murine acute lung injury. Anesthesiology 200195947–953. [DOI] [PubMed] [Google Scholar]
- 14.Wu C C, Liao M H, Chen S J.et al Terbutaline prevents circulatory failure and mitigates mortality in rodents with endotoxemia. Shock 20001460–67. [DOI] [PubMed] [Google Scholar]
- 15.Braga P C, Mancini L, Guffanti E E.et al Effects of nedocromil sodium on the oxidative burst of polymorphonuclear leukocytes: comparison with salbutamol. Drugs Exp Clin Res 19972333–38. [PubMed] [Google Scholar]
- 16.Opdahl H, Benestad H B, Nicolaysen G. Effect of β‐adrenergic agents on human neutrophil granulocyte activation with N‐formyl‐methionyl‐leucyl‐phenylalanine and phorbol myristate acetate. Pharmacol Toxicol 199372221–228. [DOI] [PubMed] [Google Scholar]
- 17.van der Poll T, Calvano S E, Kumar A.et al Epinephrine attenuates down‐regulation of monocyte tumor necrosis factor receptors during human endotoxemia. J Leukoc Biol 199761156–160. [DOI] [PubMed] [Google Scholar]
- 18.Maris N A, de Vos A F, Dessing M C.et al Antiinflammatory effects of salmeterol after inhalation of lipopolysaccharide by healthy volunteers. Am J Respir Crit Care Med 2005172878–884. [DOI] [PubMed] [Google Scholar]
- 19.Perkins G D, McAuley D F, Thickett D R.et al The β‐agonist lung injury trial (BALTI): a randomized placebo‐controlled clinical trial. Am J Respir Crit Care Med 2006173281–287. [DOI] [PubMed] [Google Scholar]
- 20.Jepsen L V, Skottun T. A rapid one‐step method for the isolation of human granulocytes from whole blood. Scand J Clin Lab Invest 198242235–238. [PubMed] [Google Scholar]
- 21.Nelson R D, Herron M J. Agarose method for human neutrophil chemotaxis. Methods Enzymol 198816250–59. [DOI] [PubMed] [Google Scholar]
- 22.Bernard G R, Artigas A, Brigham K L.et al Report of the American‐European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care 1994972–81. [DOI] [PubMed] [Google Scholar]
- 23.Perkins G D, Chatterjie S, McAuley D F.et al Role of nonbronchoscopic lavage for investigating alveolar inflammation and permeability in acute respiratory distress syndrome. Crit Care Med 20063457–64. [DOI] [PubMed] [Google Scholar]
- 24.Gunning K, Rowan K. ABC of intensive care: outcome data and scoring systems. BMJ 1999319241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzenelson R, Perel A, Berkenstadt H.et al Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med 2004321550–1554. [DOI] [PubMed] [Google Scholar]
- 26.Bateman J, Parida S K, Nash G B. Neutrophil integrin assay for clinical studies. Cell Biochem Funct 19931187–91. [DOI] [PubMed] [Google Scholar]
- 27.McAuley D F, Frank J A, Fang X.et al Clinically relevant concentrations of β2‐adrenergic agonists stimulate maximal cyclic adenosine monophosphate‐dependent airspace fluid clearance and decrease pulmonary edema in experimental acid‐induced lung injury. Crit Care Med 2004321470–1476. [DOI] [PubMed] [Google Scholar]
- 28.Harvath L, Robbins J D, Russell A A.et al cAMP and human neutrophil chemotaxis. Elevation of cAMP differentially affects chemotactic responsiveness. J Immunol 1991146224–232. [PubMed] [Google Scholar]
- 29.Silvestri M, Oddera S, Lantero S.et al Beta 2‐agonist‐induced inhibition of neutrophil chemotaxis is not associated with modification of LFA‐1 and Mac‐1 expression or with impairment of polymorphonuclear leukocyte antibacterial activity. Respir Med 199993416–423. [DOI] [PubMed] [Google Scholar]
- 30.Llewellyn‐Jones C G, Stockley R A. The effects of β 2‐agonists and methylxanthines on neutrophil function in vitro. Eur Respir J 199471460–1466. [DOI] [PubMed] [Google Scholar]
- 31.Doerschuk C M. The role of CD18‐mediated adhesion in neutrophil sequestration induced by infusion of activated plasma in rabbits. Am J Respir Cell Mol Biol 19927140–148. [DOI] [PubMed] [Google Scholar]
- 32.Kubo H, Graham L, Doyle N A.et al Complement fragment‐induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood 199892283–290. [PubMed] [Google Scholar]
- 33.Skoutelis A T, Kaleridis V, Athanassiou G M.et al Neutrophil deformability in patients with sepsis, septic shock, and adult respiratory distress syndrome. Crit Care Med 2000282355–2359. [DOI] [PubMed] [Google Scholar]
- 34.Doerschuk C M, Mizgerd J P, Kubo H.et al Adhesion molecules and cellular biomechanical changes in acute lung injury: Giles F. Filley Lecture. Chest 1999116S37–S43. [DOI] [PubMed] [Google Scholar]
- 35.Masclans J R, Barbera J A, MacNee W.et al Salbutamol reduces pulmonary neutrophil sequestration of platelet‐activating factor in humans. Am J Respir Crit Care Med 1996154529–532. [DOI] [PubMed] [Google Scholar]
- 36.Derian C K, Santulli R J, Rao P E.et al Inhibition of chemotactic peptide‐induced neutrophil adhesion to vascular endothelium by cAMP modulators. J Immunol 1995154308–317. [PubMed] [Google Scholar]
- 37.Altenburg S P, Bozza P T, Martins M A.et al Adrenergic modulation of the blood neutrophilia induced by platelet activating factor in rats. Eur J Pharmacol 199425645–49. [DOI] [PubMed] [Google Scholar]
- 38.Rainer T H.l‐selectin in health and disease. Resuscitation 200252127–141. [DOI] [PubMed] [Google Scholar]
- 39.Lee W L, Downey G P. Neutrophil activation and acute lung injury. Curr Opin Crit Care 200171–7. [DOI] [PubMed] [Google Scholar]
- 40.Mills P J, Goebel M, Rehman J.et al Leukocyte adhesion molecule expression and T cell naive/memory status following isoproterenol infusion. J Neuroimmunol 2000102137–144. [DOI] [PubMed] [Google Scholar]