Abstract
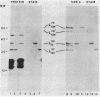
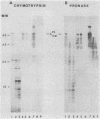
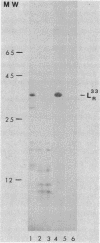
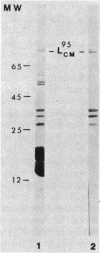
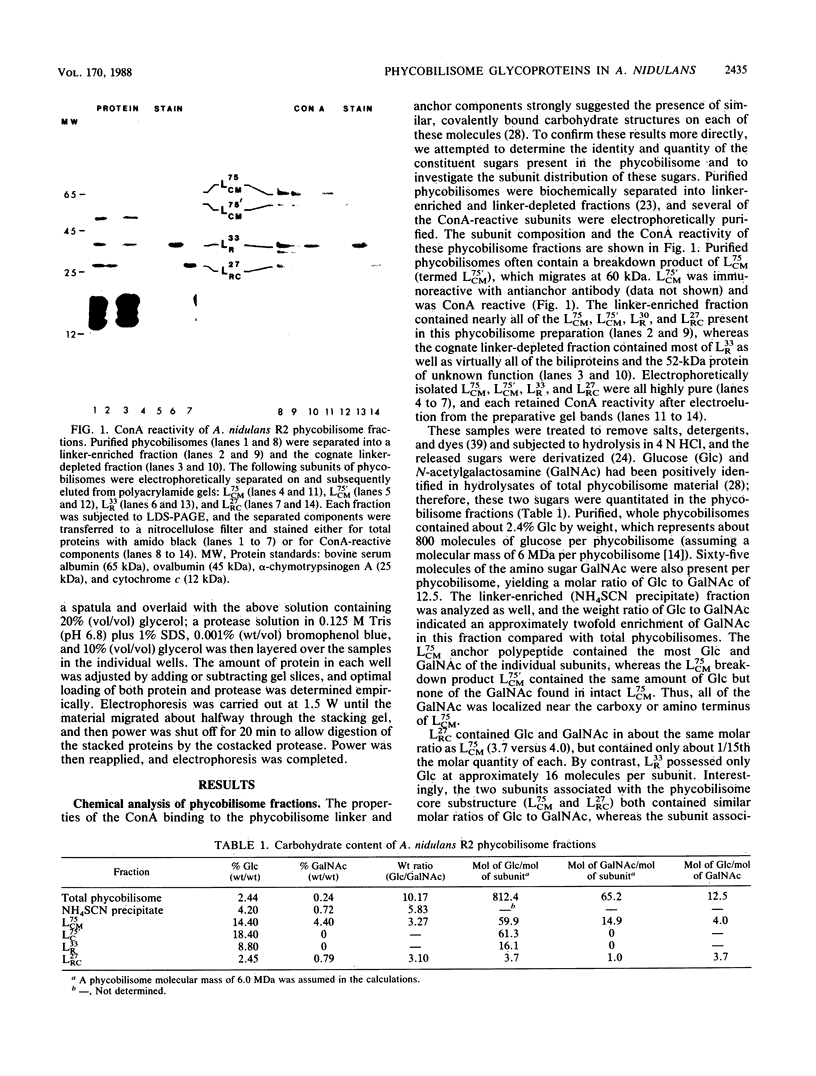
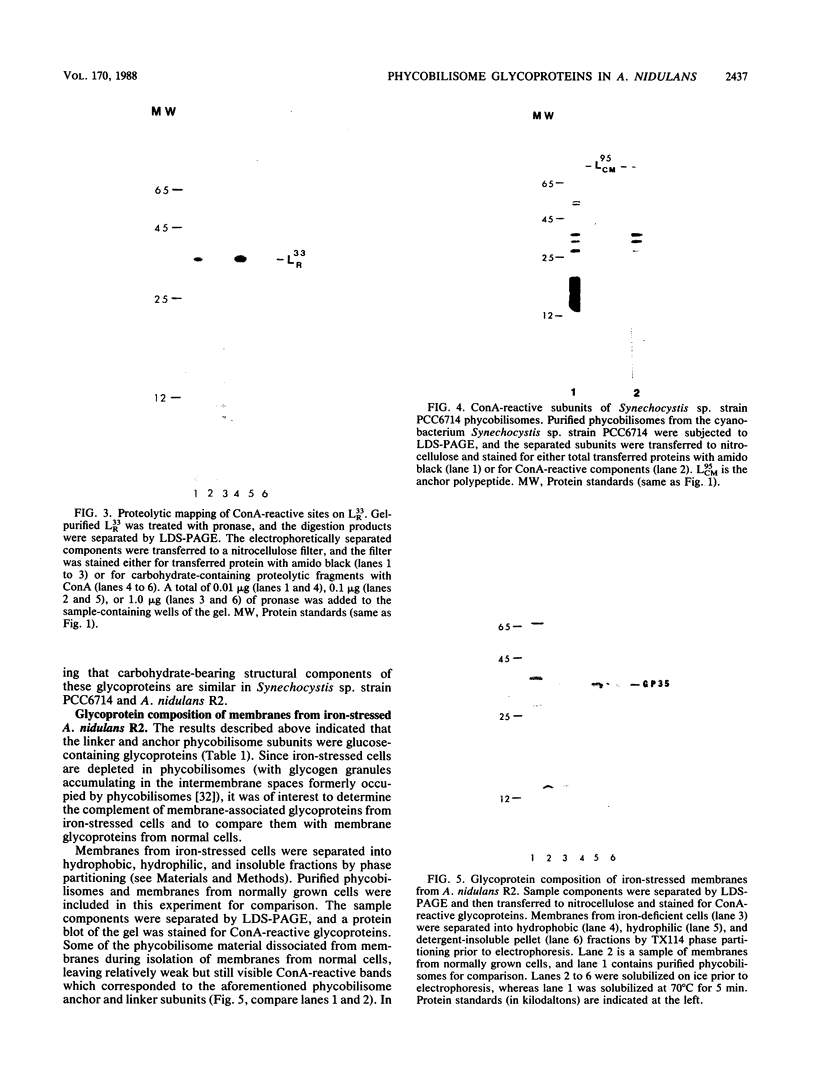
Concanavalin A-reactive linker and anchor subunits of phycobilisomes from Anacystis nidulans R2 (H. C. Riethman, T. P. Mawhinney, and L. A. Sherman, FEBS Lett. 215:209-214, 1987) were purified electrophoretically and analyzed for carbohydrate composition and quantity. Different quantities of glucose and N-acetylgalactosamine were found on the concanavalin A-reactive subunits analyzed. Proteolytic analysis of the purified subunits suggested that small regions of the 33- and 27-kilodalton linker polypeptides previously shown to be important for in vitro phycobilisome assembly contained the concanavalin A-reactive carbohydrates present on these subunits. The linker and anchor subunits from the morphologically different phycobilisome of Synechocystis sp. strain PCC6714 were also shown to be concanavalin A reactive. Membranes from iron-starved Anacystis nidulans, which lack assembled phycobilisomes and are associated with glycogen deposits, were shown to be depleted of linker and anchor proteins and to accumulate very large quantities of a concanavalin A-reactive, extrinsic membrane glycoprotein. We suggest that this iron stress-induced glycoprotein is associated with the glycogen deposits on the thylakoid surface and that the glycosylation of phycobilisome linker and anchor subunits is involved in the physiological regulation of phycobilisome assembly and degradation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. K., Eiserling F. A. Asymmetrical core structure in phycobilisomes of the cyanobacterium Synechocystis 6701. J Mol Biol. 1986 Oct 5;191(3):441–451. doi: 10.1016/0022-2836(86)90139-7. [DOI] [PubMed] [Google Scholar]
- Belknap W. R., Haselkorn R. Cloning and light regulation of expression of the phycocyanin operon of the cyanobacterium Anabaena. EMBO J. 1987 Apr;6(4):871–884. doi: 10.1002/j.1460-2075.1987.tb04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bricker T. M., Sherman L. A. Triton X-114 phase fractionation of membrane proteins of the cyanobacterium Anacystis nidulans R2. Arch Biochem Biophys. 1984 Nov 15;235(1):204–211. doi: 10.1016/0003-9861(84)90269-8. [DOI] [PubMed] [Google Scholar]
- Bullerjahn G. S., Riethman H. C., Sherman L. A. Organization of the thylakoid membrane from the heterotrophic cyanobacterium, Aphanocapsa 6714. Biochim Biophys Acta. 1985 Nov 27;810(2):148–157. doi: 10.1016/0005-2728(85)90130-6. [DOI] [PubMed] [Google Scholar]
- Clegg J. C. Glycoprotein detection in nitrocellulose transfers of electrophoretically separated protein mixtures using concanavalin A and peroxidase: application to arenavirus and flavivirus proteins. Anal Biochem. 1982 Dec;127(2):389–394. doi: 10.1016/0003-2697(82)90192-0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Frösch D., Westphal C., Böhme H. Improved preservation of glycogen in unfixed cyanobacteria embedded at -82 degrees C in nanoplast. J Histochem Cytochem. 1987 Jan;35(1):119–121. doi: 10.1177/35.1.3098832. [DOI] [PubMed] [Google Scholar]
- Füglistaller P., Suter F., Zuber H. Linker polypeptides of the phycobilisome from the cyanobacterium Mastigocladus laminosus. II. Amino-acid sequences and functions. Biol Chem Hoppe Seyler. 1986 Jul;367(7):615–626. doi: 10.1515/bchm3.1986.367.2.615. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Influence of Iron Deprivation on the Membrane Composition of Anacystis nidulans. Plant Physiol. 1984 Jan;74(1):90–95. doi: 10.1104/pp.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax T. L., Conley P. B., Schilling J., Grossman A. R. Isolation and characterization of light-regulated phycobilisome linker polypeptide genes and their transcription as a polycistronic mRNA. J Bacteriol. 1987 Jun;169(6):2675–2684. doi: 10.1128/jb.169.6.2675-2684.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Quaternary interactions in the Synechococcus 6301 phycobilisome core as revealed by partial tryptic digestion and circular dichroism studies. J Biol Chem. 1983 Jul 25;258(14):8708–8713. [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Structure of the 18 S core-rod subassembly of the Synechococcus 6301 phycobilisome. J Biol Chem. 1983 Jan 25;258(2):894–901. [PubMed] [Google Scholar]
- Lundell D. J., Williams R. C., Glazer A. N. Molecular architecture of a light-harvesting antenna. In vitro assembly of the rod substructures of Synechococcus 6301 phycobilisomes. J Biol Chem. 1981 Apr 10;256(7):3580–3592. [PubMed] [Google Scholar]
- Lundell D. J., Yamanaka G., Glazer A. N. A terminal energy acceptor of the phycobilisome: the 75,000-dalton polypeptide of Synechococcus 6301 phycobilisomes--a new biliprotein. J Cell Biol. 1981 Oct;91(1):315–319. doi: 10.1083/jcb.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS J., KRATZ W. A. Relation between pigment content and photosynthetic characteristics in a blue-green algae. J Gen Physiol. 1955 Sep 20;39(1):11–22. doi: 10.1085/jgp.39.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney T. P. Simultaneous determination of N-acetylglucosamine, N-acetylgalactosamine, N-acetylglucosaminitol and N-acetylgalactosaminitol by gas-liquid chromatography. J Chromatogr. 1986 Jan 3;351(1):91–102. doi: 10.1016/s0021-9673(01)83475-0. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Riethman H. C., Sherman L. A. Organization of pigment proteins in the photosystem II complex of the cyanobacterium Anacystis nidulans R2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6903–6907. doi: 10.1073/pnas.82.20.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Mawhinney T. P., Sherman L. A. Phycobilisome-associated glycoproteins in the cyanobacterium Anacystis nidulans R 2. FEBS Lett. 1987 May 11;215(2):209–214. doi: 10.1016/0014-5793(87)80147-3. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Sherman D. M., Sherman L. A. Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):393–401. doi: 10.1128/jb.156.1.393-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens S. E., Paone D. A. Accumulation of Cyanophycin Granules as a Result of Phosphate Limitation in Agmenellum quadruplicatum. Plant Physiol. 1981 Apr;67(4):716–719. doi: 10.1104/pp.67.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., McIntosh M. A., Robbins J., Wise K. S. Cloned genomic DNA sequences from Mycoplasma hyorhinis encoding antigens expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4154–4158. doi: 10.1073/pnas.80.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N. Cyanobacterial phycobilisomes. Role of the linker polypeptides in the assembly of phycocyanin. J Biol Chem. 1982 Apr 10;257(7):3429–3433. [PubMed] [Google Scholar]
- Zilinskas B. A., Howell D. A. The immunologically conserved phycobilisome-thylakoid linker polypeptide. Plant Physiol. 1986 Apr;80(4):829–833. doi: 10.1104/pp.80.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]