Abstract
DNA sequence analysis of the complete M6 protein gene revealed 19 hydrophobic amino acids at the C terminus which could act as a membrane anchor and an adjacent proline- and glycine-rich region likely to be located in the cell wall. To define this region within the cell wall and its role in attaching the molecule to the cell, we isolated the cell-associated fragment of the M protein. Assuming that the cell-associated region of the M protein would be embedded within the wall and thus protected from trypsin digestion, cells were digested with this enzyme, and the wall-associated M protein fragment was released by phage lysin digestion of the peptidoglycan. With antibody probes prepared to synthetic peptides of C-terminal sequences, a cell wall-associated M protein fragment (molecular weight, 16,000) was identified and purified. Amino acid sequence analysis placed the N terminus of the 16,000-molecular-weight fragment at residue 298 within the M sequence. Amino acid composition of this peptide was consistent with a C-terminal sequence lacking the membrane anchor. Antibody studies of nitrous acid-extracted whole bacteria suggested that, in addition to the peptidoglycan-associated region, a 65-residue helical segment of the C-terminal domain of the M protein is embedded within the carbohydrate moiety of the cell wall. Since no detectable amino sugars were associated with the wall-associated fragment, the C-terminal region of the M6 molecule is likely to be intercalated within the cross-linked peptidoglycan and not covalently linked to it. Because the C-terminal region of the M molecule is highly homologous to the C-terminal end of protein A from staphylococci and protein G from streptococci, it is likely that the mechanism of attachment of these proteins to the cell wall is conserved.
Full text
PDF


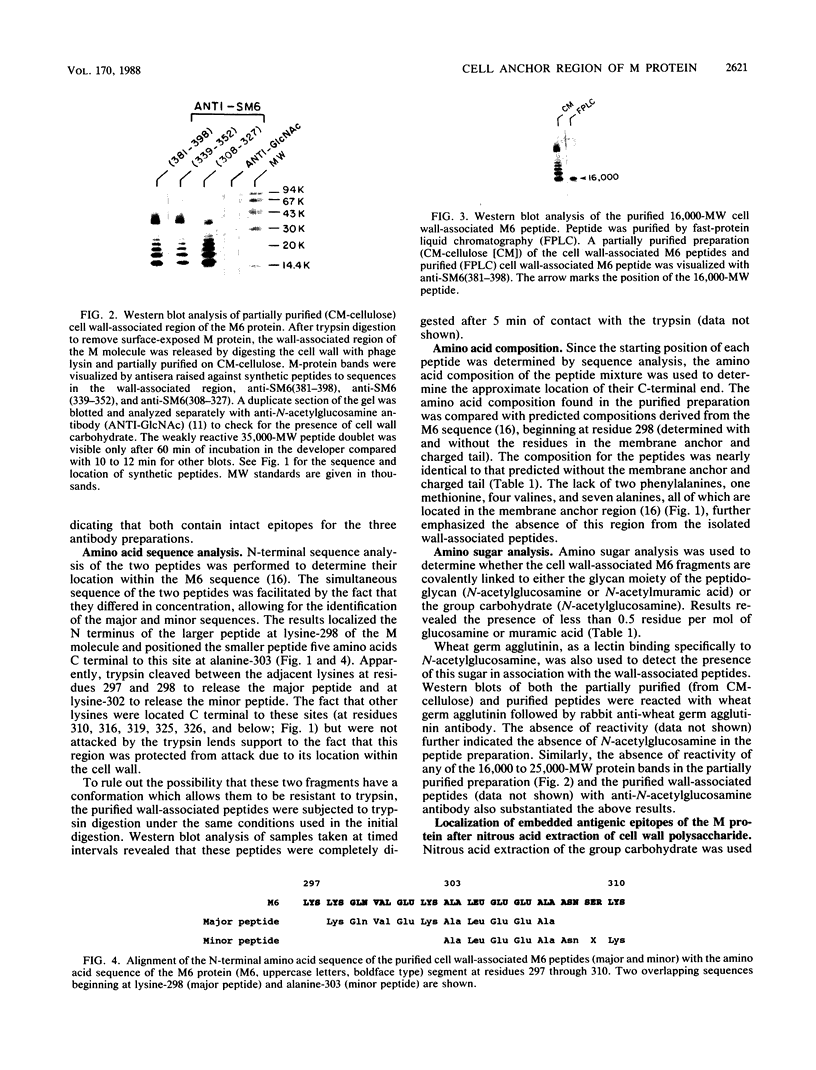

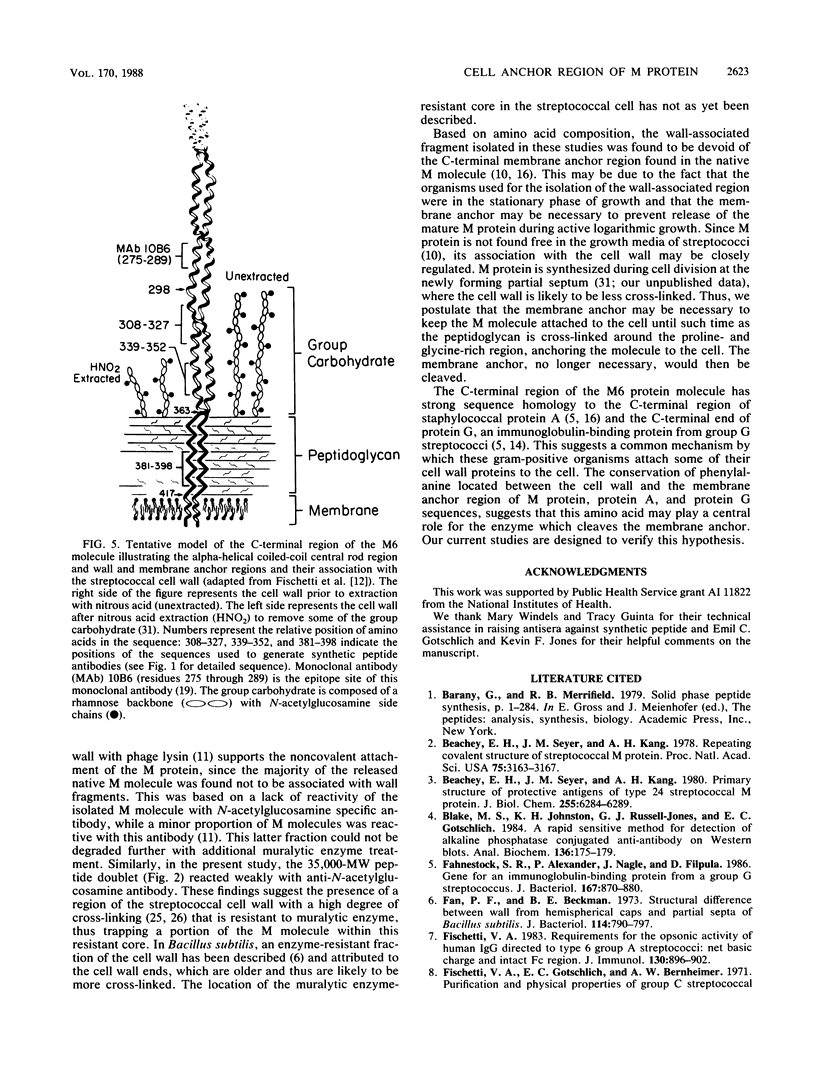

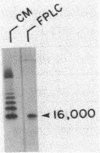
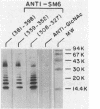
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Seyer J. M., Kang A. H. Primary structure of protective antigens of type 24 streptococcal M protein. J Biol Chem. 1980 Jul 10;255(13):6284–6289. [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M., Kang A. H. Repeating covalent structure of streptococcal M protein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3163–3167. doi: 10.1073/pnas.75.7.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Fahnestock S. R., Alexander P., Nagle J., Filpula D. Gene for an immunoglobulin-binding protein from a group G streptococcus. J Bacteriol. 1986 Sep;167(3):870–880. doi: 10.1128/jb.167.3.870-880.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E. Structural difference between walls from hemispherical caps and partial septa of Bacillus subtilis. J Bacteriol. 1973 May;114(2):790–797. doi: 10.1128/jb.114.2.790-797.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Parry D. A., Trus B. L., Hollingshead S. K., Scott J. R., Manjula B. N. Conformational characteristics of the complete sequence of group A streptococcal M6 protein. Proteins. 1988;3(1):60–69. doi: 10.1002/prot.340030106. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Requirements for the opsonic activity of human IgG directed to type 6 group A streptococci: net basic charge and intact Fc region. J Immunol. 1983 Feb;130(2):896–902. [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Guss B., Eliasson M., Olsson A., Uhlén M., Frej A. K., Jörnvall H., Flock J. I., Lindberg M. Structure of the IgG-binding regions of streptococcal protein G. EMBO J. 1986 Jul;5(7):1567–1575. doi: 10.1002/j.1460-2075.1986.tb04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss B., Uhlén M., Nilsson B., Lindberg M., Sjöquist J., Sjödahl J. Region X, the cell-wall-attachment part of staphylococcal protein A. Eur J Biochem. 1984 Jan 16;138(2):413–420. doi: 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. A highly conserved region present in transcripts encoding heterologous M proteins of group A streptococci. Infect Immun. 1987 Dec;55(12):3237–3239. doi: 10.1128/iai.55.12.3237-3239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Jones K. F., Khan S. A., Erickson B. W., Hollingshead S. K., Scott J. R., Fischetti V. A. Immunochemical localization and amino acid sequences of crossreactive epitopes within the group A streptococcal M6 protein. J Exp Med. 1986 Oct 1;164(4):1226–1238. doi: 10.1084/jem.164.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. F., Manjula B. N., Johnston K. H., Hollingshead S. K., Scott J. R., Fischetti V. A. Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J Exp Med. 1985 Mar 1;161(3):623–628. doi: 10.1084/jem.161.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- Manjula B. N., Acharya A. S., Mische S. M., Fairwell T., Fischetti V. A. The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J Biol Chem. 1984 Mar 25;259(6):3686–3693. [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Studies on group A streptococcal M-proteins: purification of type 5 M-protein and comparison of its amino terminal sequence with two immunologically unrelated M-protein molecules. J Immunol. 1980 Jan;124(1):261–267. [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Tropomyosin-like seven residue periodicity in three immunologically distinct streptococal M proteins and its implications for the antiphagocytic property of the molecule. J Exp Med. 1980 Mar 1;151(3):695–708. doi: 10.1084/jem.151.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERUTZ M. F. New x-ray evidence on the configuration of polypeptide chains. Nature. 1951 Jun 30;167(4261):1053–1054. doi: 10.1038/1671053a0. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Hollingshead S. K., Fischetti V. A. Homologous regions within M protein genes in group A streptococci of different serotypes. Infect Immun. 1986 May;52(2):609–612. doi: 10.1128/iai.52.2.609-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodahl J. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur J Biochem. 1977 Mar 1;73(2):343–351. doi: 10.1111/j.1432-1033.1977.tb11324.x. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Movitz J., Johansson I. B., Hjelm H. Localization of protein A in the bacteria. Eur J Biochem. 1972 Oct 17;30(1):190–194. doi: 10.1111/j.1432-1033.1972.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]