Abstract
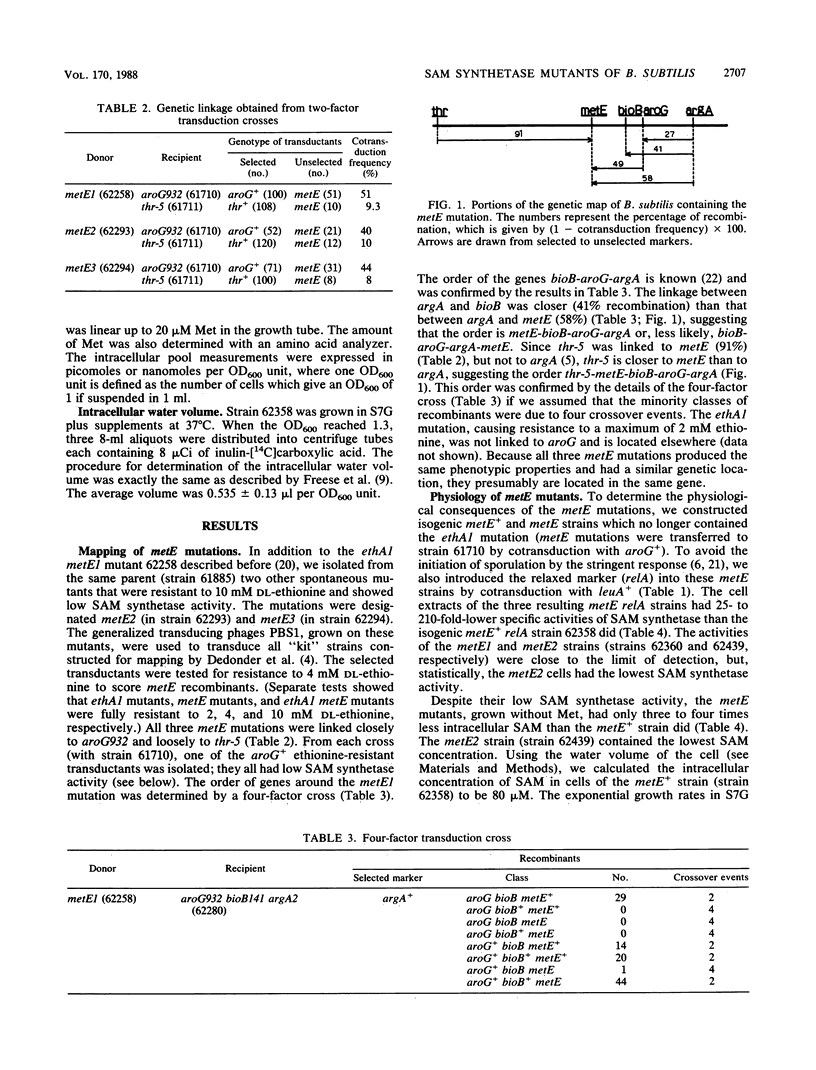
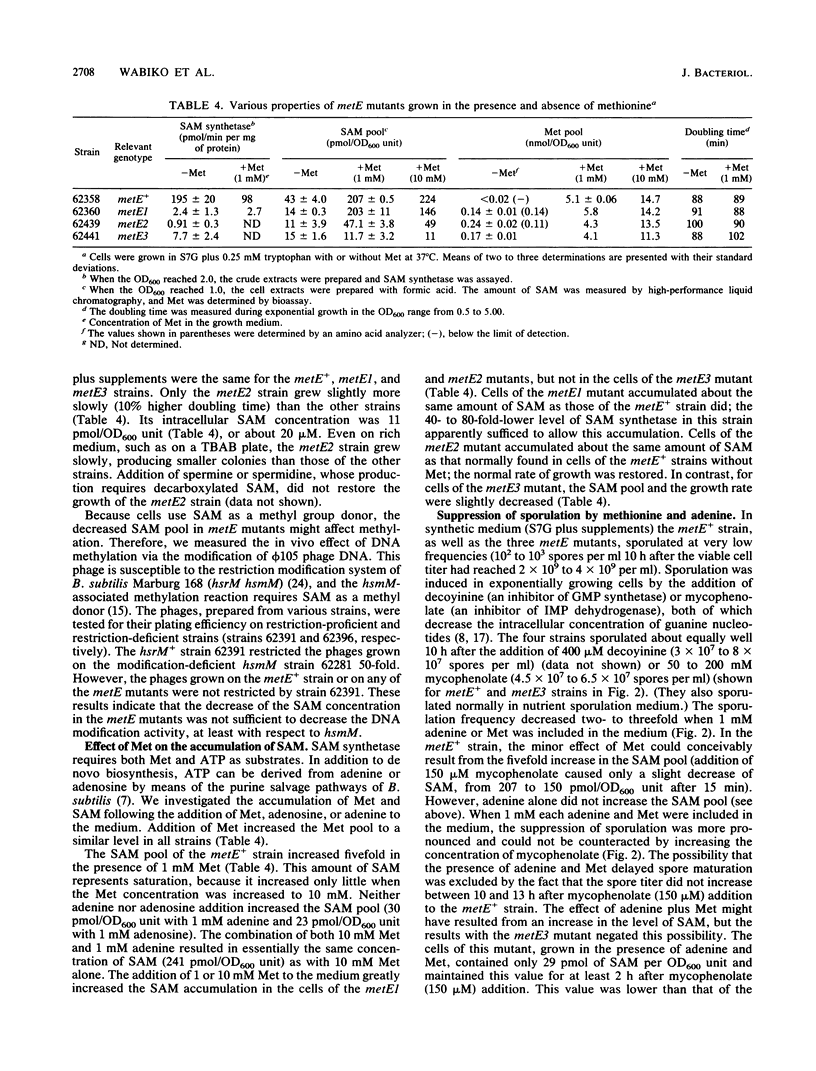
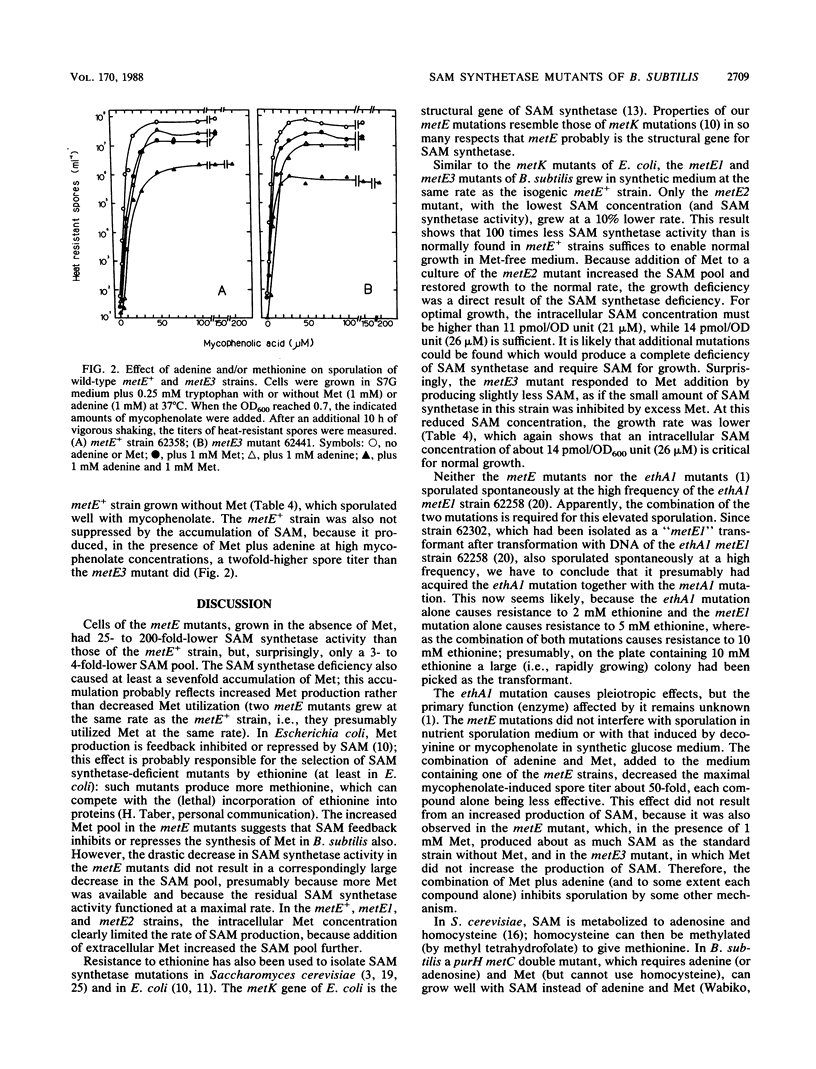
Three metE mutations of Bacillus subtilis, which cause cells to have a 25- to 200-fold decrease in L-methionine S-adenosyltransferase (EC 2.5.1.6) activity, were mapped between bioB and thr. The corresponding three metE mutants contained three- to fourfold less intracellular S-adenosylmethionine (SAM) but at least sevenfold more methionine than the metE+ strain when grown in synthetic medium. This indicates a strong feedback control of SAM on its synthesis. However, only the metE2 strain, with the lowest SAM concentration, grew at a slightly lower rate than the parent, which showed that an intracellular concentration of about 25 microM SAM was critical for growth at the normal rate. Neither DNA methylation (measured by bacteriophage luminal diameter 105 restriction) nor sporulation was affected at this low SAM concentration. Addition of methionine to the growth medium caused an increase in the pool of SAM in some but not all metE mutants. Coaddition of adenine did not change this result. However, the extent of sporulation (induced by mycophenolic acid) was decreased 50-fold in all mutants by the addition of methionine and adenine. Therefore, the combination of methionine and adenine suppresses sporulation regardless of whether it causes an increase in the level of SAM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. R., Orrego C., Wabiko H., Freese E. An ethA mutation in Bacillus subtilis 168 permits induction of sporulation by ethionine and increases DNA modification of bacteriophage phi 105. J Bacteriol. 1986 Apr;166(1):1–8. doi: 10.1128/jb.166.1.1-8.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y. S-adenosyl methionine requiring mutants in Saccharomyces cerevisiae: evidences for the existence of two methionine adenosyl transferases. Mol Gen Genet. 1978 Jul 11;163(2):153–167. doi: 10.1007/BF00267406. [DOI] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Endo T., Ishihara H., Freese E. Properties of a Bacillus subtilis mutant able to sporulate continually during growth in synthetic medium. J Gen Microbiol. 1983 Jan;129(1):17–30. doi: 10.1099/00221287-129-1-17. [DOI] [PubMed] [Google Scholar]
- Endo T., Uratani B., Freese E. Purine salvage pathways of Bacillus subtilis and effect of guanine on growth of GMP reductase mutants. J Bacteriol. 1983 Jul;155(1):169–179. doi: 10.1128/jb.155.1.169-179.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E. B., Olempska-Beer Z., Hartig A., Freese E. Initiation of meiosis and sporulation of Saccharomyces cerevisiae by sulfur or guanine deprivation. Dev Biol. 1984 Apr;102(2):438–451. doi: 10.1016/0012-1606(84)90209-4. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Su C. H., Holloway C. T. S-Adenosylmethionine synthetase deficient mutants of Escherichia coli K-12 with impaired control of methionine biosynthesis. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1120–1126. doi: 10.1016/0006-291x(70)90355-4. [DOI] [PubMed] [Google Scholar]
- Günthert U., Jentsch S., Freund M. Restriction and modification in Bacillus subtilis: two DNA methyltransferases with BsuRI specificity. II. Catalytic properties, substrate specificity, and mode of action. J Biol Chem. 1981 Sep 10;256(17):9346–9351. [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Isolation of a metK mutant with a temperature-sensitive S-adenosylmethionine synthetase. J Bacteriol. 1977 Dec;132(3):832–840. doi: 10.1128/jb.132.3.832-840.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Ando T., Saito H. Host-controlled modification and restriction in Bacillus subtilis: Bsu 168-system and BsuR-system in B. subtilis 168. Mol Gen Genet. 1979 Feb 26;170(2):123–127. doi: 10.1007/BF00337786. [DOI] [PubMed] [Google Scholar]
- Jentsch S. Restriction and modification in Bacillus subtilis: sequence specificities of restriction/modification systems BsuM, BsuE, and BsuF. J Bacteriol. 1983 Nov;156(2):800–808. doi: 10.1128/jb.156.2.800-808.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen R. C., Moore K., Yall I. Uptake and utilization of S-adenosyl-L-methionine and S-adenosyl-L-homocysteine in an adenine mutant of Saccharomyces cerevisiae. J Bacteriol. 1969 May;98(2):629–636. doi: 10.1128/jb.98.2.629-636.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopez J. M., Marks C. L., Freese E. The decrease of guanine nucleotides initiates sporulation of Bacillus subtilis. Biochim Biophys Acta. 1979 Oct 4;587(2):238–252. doi: 10.1016/0304-4165(79)90357-x. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Spence K. D. Methionine adenosyltransferase and ethionine resistance in Saccharomyces cerevisiae. J Bacteriol. 1972 Sep;111(3):778–783. doi: 10.1128/jb.111.3.778-783.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Freese E. A decrease in S-adenosylmethionine synthetase activity increases the probability of spontaneous sporulation. J Bacteriol. 1982 Oct;152(1):400–410. doi: 10.1128/jb.152.1.400-410.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Kandala J. C., Freese E. Initiation of Bacillus subtilis sporulation by the stringent response to partial amino acid deprivation. J Biol Chem. 1981 Jul 10;256(13):6866–6875. [PubMed] [Google Scholar]
- Pai C. H. Genetics of biotin biosynthesis in Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):1–8. doi: 10.1128/jb.121.1.1-8.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi G., Sora S., Ciferri O. Production of amino acids by analog-resistant mutants of the cyanobacterium Spirulina platensis. J Bacteriol. 1981 Sep;147(3):1002–1007. doi: 10.1128/jb.147.3.1002-1007.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Ando T. Host controlled modification and restriction in Bacillus subtilis. Mol Gen Genet. 1974;131(4):275–280. doi: 10.1007/BF00264858. [DOI] [PubMed] [Google Scholar]
- Spence K. D., Parks L. W., Shapiro S. K. Dominant mutation for ethionine resistance in Saccharomyces cerevisae. J Bacteriol. 1967 Nov;94(5):1531–1537. doi: 10.1128/jb.94.5.1531-1537.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton M., Edlin G. Isolation and characterization of an RNA relaxed mutant of B. subtilis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):583–588. doi: 10.1016/s0006-291x(72)80179-7. [DOI] [PubMed] [Google Scholar]


