Abstract
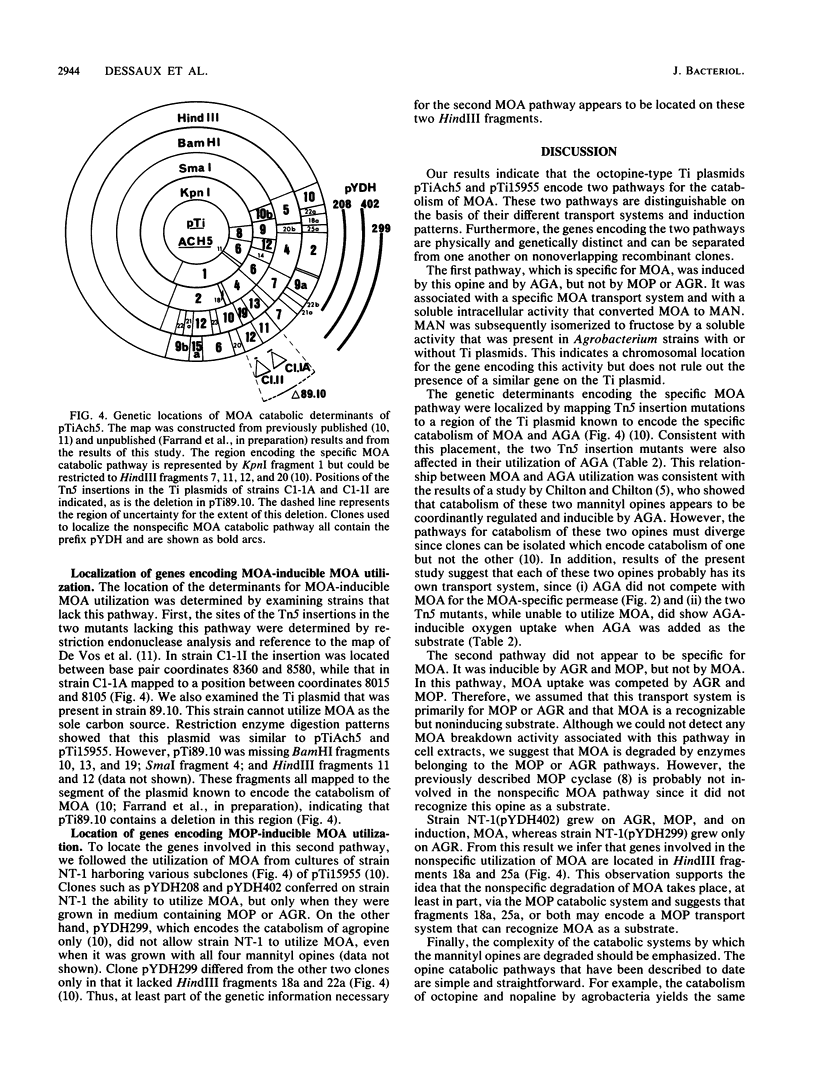
Octopine-type strains of Agrobacterium tumefaciens degrade the opine mannopinic acid through a specific pathway which involves cleavage of the molecule at the C--N bond between the amino acid and the sugar moieties. Mannose was identified as a product of the reaction. This pathway was inducible by mannopinic and agropinic acids, but not by mannopine or agropine, the two other mannityl opines. The transport system for this pathway appeared to be specific for mannopinic acid. A second, nonspecific pathway for mannopinic acid degradation was also identified. This involved some of the catabolic functions associated with the metabolism of mannopine and agropine. This second pathway was inducible by mannopine and agropine but not by mannopinic or agropinic acids. The transport system for this pathway appeared to have a broad specificity. Transposon Tn5 insertion mutants affected in the specific catabolic pathway were isolated and analyzed. These mutants continued to catabolize mannopine and agropine. Both mapped to a region of the Ti plasmid previously shown to be associated with the catabolism of mannopinic acid. Restriction enzyme analysis of the Ti plasmid from strain 89.10, an octopine strain that is naturally unable to utilize mannopinic acid, showed a deletion in this same region encoding the specific mannopinic acid degradation pathway. Analysis of recombinant clones showed that the second, nonspecific pathway was encoded in a region of the Ti plasmid associated with mannopine and agropine catabolism. This region shared no structural overlap with the segment of the plasmid encoding the specific mannopinic acid degradative pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang C. C., Chen C. M., Adams B. R., Trost B. M. Leucinopine, a characteristic compound of some crown-gall tumors. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3573–3576. doi: 10.1073/pnas.80.12.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton W. S., Chilton M. D. Mannityl opine analogs allow isolation of catabolic pathway regulatory mutants. J Bacteriol. 1984 May;158(2):650–658. doi: 10.1128/jb.158.2.650-658.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton W. S., Tempé J., Matzke M., Chilton M. D. Succinamopine: a new crown gall opine. J Bacteriol. 1984 Feb;157(2):357–362. doi: 10.1128/jb.157.2.357-362.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greve H., Decraemer H., Seurinck J., Van Montagu M., Schell J. The functional organization of the octopine Agrobacterium tumefaciens plasmid pTiB6s3. Plasmid. 1981 Sep;6(2):235–248. doi: 10.1016/0147-619x(81)90069-x. [DOI] [PubMed] [Google Scholar]
- De Vos G., De Beuckeleer M., Van Montagu M., Schell J. Restriction endonuclease mapping of the octopine tumor-inducing plasmid pTiAch5 of Agrobacterium tumefaciens. Plasmid. 1981 Sep;6(2):249–253. doi: 10.1016/0147-619x(81)90070-6. [DOI] [PubMed] [Google Scholar]
- Dessaux Y., Guyon P., Farrand S. K., Petit A., Tempé J. Agrobacterium Ti and Ri plasmids specify enzymic lactonization of mannopine to agropine. J Gen Microbiol. 1986 Sep;132(9):2549–2559. doi: 10.1099/00221287-132-9-2549. [DOI] [PubMed] [Google Scholar]
- Dessaux Y., Petit A., Tempé J., Demarez M., Legrain C., Wiame J. M. Arginine catabolism in Agrobacterium strains: role of the Ti plasmid. J Bacteriol. 1986 Apr;166(1):44–50. doi: 10.1128/jb.166.1.44-50.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
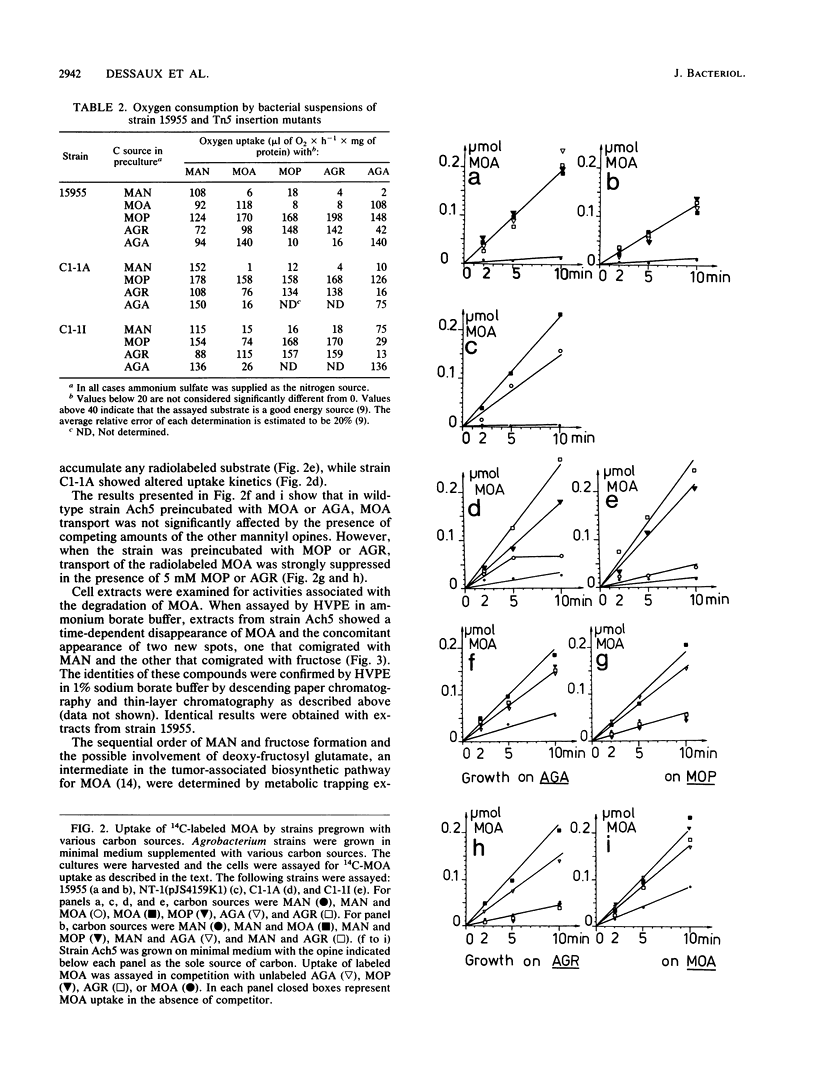
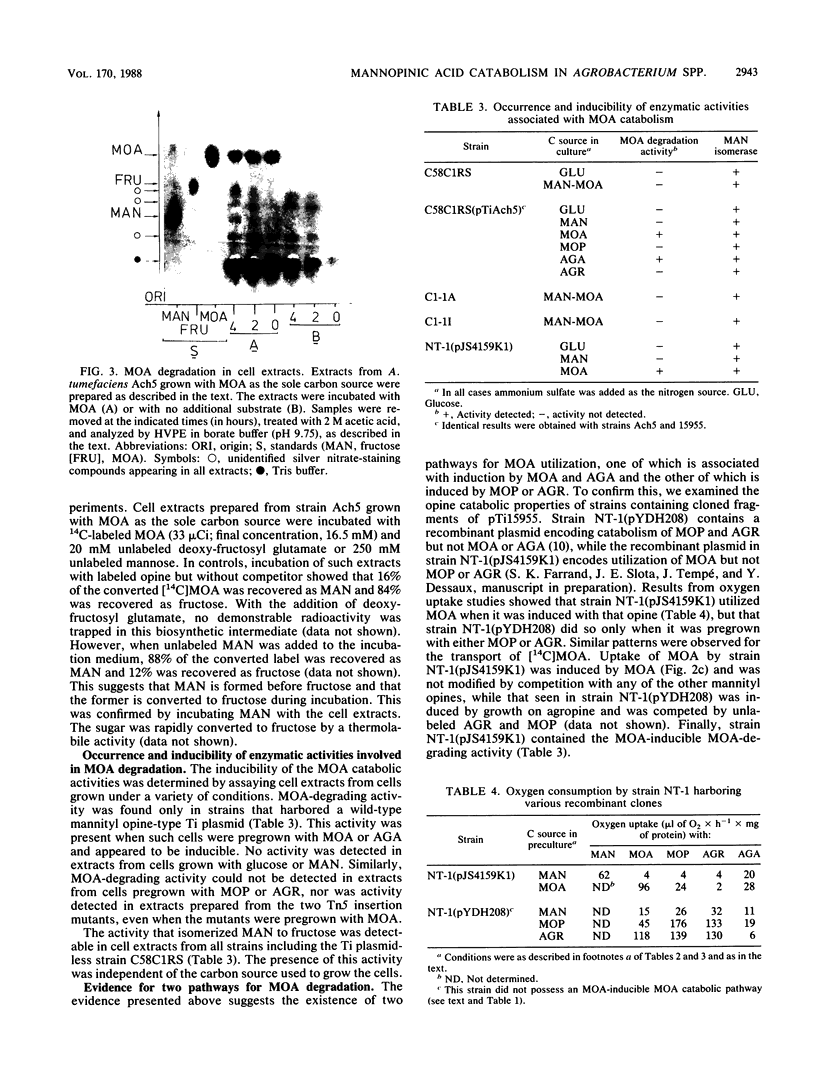
- Dessaux Y., Tempé J., Farrand S. K. Genetic analysis of mannityl opine catabolism in octopine-type Agrobacterium tumefaciens strain 15955. Mol Gen Genet. 1987 Jun;208(1-2):301–308. doi: 10.1007/BF00330457. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B., Gordon M. P., Nester E. W., Aronson A. I. Transcription of the Agrobacterium Ti plasmid in the bacterium and in crown gall tumors. Plasmid. 1981 Jul;6(1):17–29. doi: 10.1016/0147-619x(81)90051-2. [DOI] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A., Manigault P., Tempé J. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature. 1977 Feb 10;265(5594):560–561. doi: 10.1038/265560a0. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. J., Heycke N., Banfalvi Z., Tate M. E., de Bruijn F., Kondorosi A., Tempé J., Schell J. Genes for the catabolism and synthesis of an opine-like compound in Rhizobium meliloti are closely linked and on the Sym plasmid. Proc Natl Acad Sci U S A. 1987 Jan;84(2):493–497. doi: 10.1073/pnas.84.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon F., Deblaere R., Leemans J., Hernalsteens J. P., Van Montagu M., Schell J. Genetic identification of functions of TR-DNA transcripts in octopine crown galls. EMBO J. 1984 Jan;3(1):141–146. doi: 10.1002/j.1460-2075.1984.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell J., Van Montagu M., De Beuckeleer M., De Block M., Depicker A., De Wilde M., Engler G., Genetello C., Hernalsteens J. P., Holsters M. Interactions and DNA transfer between Agrobacterium tumefaciens, the Ti-plasmid and the plant host. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):251–266. doi: 10.1098/rspb.1979.0026. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tempé J., Guyon P., Petit A., Ellis J. G., Tate M. E., Kerr A. Préparation et propriétés de nouveaux substrats cataboliques pour deux types de plasmides oncogènes d'Agrobacterium tumefaciens. C R Seances Acad Sci D. 1980 May 5;290(17):1173–1176. [PubMed] [Google Scholar]
- Tremblay G., Gagliardo R., Chilton W. S., Dion P. Diversity among Opine-Utilizing Bacteria: Identification of Coryneform Isolates. Appl Environ Microbiol. 1987 Jul;53(7):1519–1524. doi: 10.1128/aem.53.7.1519-1524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]