Abstract
Membrane tubules of uniform diameter (60–80 nm) and various lengths (up to several micrometers) emanate from elements of the Golgi stack and trans Golgi network (TGN). These organelle membrane tubules are thought to be involved in membrane trafficking and maintenance of Golgi/TGN architecture. The number of these tubules, and their frequency of formation, can be greatly enhanced by the fungal metabolite brefeldin A (BFA), an inhibitor of Golgi/TGN-associated coated vesicle formation. We show here that BFA stimulation of Golgi and TGN membrane tubulation, and the resultant retrograde transport of resident Golgi enzymes to the endoplasmic reticulum, was potently inhibited by a number of membrane-permeant antagonists of phospholipase A2 (PLA2; EC 3.1.1.4) activity. In addition, PLA2 inhibitors on their own caused a reversible fragmentation of the Golgi complex into juxtanuclear, stacked cisternal elements. We conclude from these observations that tubulation of Golgi complex and TGN membranes requires a PLA2 activity, and that this activity may participate not only in Golgi tubule-mediated retrograde trafficking to the endoplasmic reticulum, but also in the maintenance of Golgi complex architecture.
The Golgi complex and trans Golgi network (TGN) are highly organized and morphologically complicated intracellular organelles that serve as a hub of membrane trafficking in both the secretory (biosynthetic) and endocytic pathways (1). Our understanding of the mechanisms by which membrane-bounded cargo traffics to and from the Golgi complex and TGN has been significantly advanced by extensive morphological, biochemical, and genetic studies, which have established an important role for various types of coated vesicles (COPI, COPII, and clathrin) as mediators of intracellular membrane trafficking (2, 3). In addition, morphological studies over the past 30 years have led some to speculate that organelle membrane tubules may also participate in trafficking. For example, membrane tubules, uniformly 60–80 nm in diameter, but variable in length (up to several micrometers), have been seen to extend from the Golgi complex outwards into the cytoplasm and to form direct membrane continuities between otherwise spatially separate cisternal stacks (4–9).
Several years ago, the study of organelle membrane tubules was stimulated by the finding that brefeldin A (BFA), a fungal metabolite that inhibits the formation of COPI- and clathrin-coated vesicles from Golgi and TGN membranes (10), respectively, significantly enhanced the formation of tubules from these same organelles (10–13). In the case of the Golgi complex, BFA induced the tubule-mediated retrograde movement of resident enzymes back to the endoplasmic reticulum (ER) (11, 12), whereas TGN tubules fused with early endosomes (12, 13). Studies with BFA suggested, therefore, that membrane tubules may serve as important mediators of trafficking events between various organelles. Support for this idea has recently emerged from time-lapse fluorescence imaging studies, which demonstrated that, at steady state, membrane tubules are continuously forming and detaching from the Golgi complex (14).
The molecular mechanisms that lead directly or indirectly to the formation of Golgi and TGN membrane tubules are only beginning to be understood. For example, BFA-stimulated tubulation and retrograde transport are greatly facilitated by, but not absolutely dependent upon, microtubules and the microtubule-associated motor protein, kinesin (10–13, 15). However, because the formation of short (<1 μm long) membrane tubules (13), and subsequent retrograde trafficking to the ER, still occurs even when microtubules have been depolymerized, albeit more slowly (10, 14), other cytoplasmic factors would also appear to be involved in tubule formation. Support for this idea was obtained by showing that the formation of Golgi membrane tubules could be reconstituted in vitro in a microtubule-independent, cytosolic protein-dependent manner (16, 17). These studies demonstrated that in vitro tubulation required relatively low concentrations of a highly enriched, heat-labile fraction of bovine brain cytosol, suggesting that the activity may function enzymatically.
A clue to possible cytoplasmic activities that may directly or indirectly regulate Golgi membrane tubulation came from studies showing that BFA-stimulated tubulation of both Golgi complex and endosomal membranes was potently inhibited by calmodulin antagonists (18). Moreover, these same antagonists inhibited receptor recycling from various endosomal compartments (18, 19), consistent with the idea that organelle membrane tubules play a role in intracellular trafficking. Given calmodulin’s typical role as a regulatory protein (20), it is unlikely to be directly responsible for the formation of membrane tubules. Of the many possible calmodulin targets, one class of enzymes, the cytoplasmic phospholipases, was particularly intriguing because they have long been thought to be involved in membrane remodeling and signal transduction (21, 22), some are regulated by calmodulin (23), and an extensive pharmacology of phospholipases exists (24), thereby providing tools for a pharmacological test of the potential role of phospholipases in membrane tubulation. We report here that a wide variety of general and specific membrane-permeant phospholipase inhibitors potently and rapidly inhibit BFA-stimulated tubulation of Golgi complex and TGN membranes in living cells. Together, the results suggest that an intracellular phospholipase A2 (PLA2; EC 3.1.1.4) activity may be intimately involved in membrane tubulation and in the trafficking events these tubules may mediate.
MATERIALS AND METHODS
Materials.
Phospholipase and other inhibitors were obtained from the following sources: N-(p-amylcinnamoyl)anthranilic acid (ACA), arachidonyl trifluoromethyl ketone (AACOCF3), aristolochic acid (ARA), baicalein, 7,7-dimethyleicosadienoic acid (DEDA), eicosatriynoic acid (ETI), 2-(p-amylcinnamoyl)amino-4-cholorobenzoic acid (ONO-RS-082), palmityl trifluoromethyl ketone (PACOCF3), and prostaglandin E2 (PGE2) were from Biomol (Plymouth Meeting, PA); E-6-(bromomethylene)tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one (HELSS, or bromoenol lactone, BEL) was originally synthesized in the Katzenellenbogen laboratory (25) and later was obtained from Biomol; quinacrine, dibucaine, esculetin, ibuprofen, indomethacin, p-bromophenacyl bromide, and all other common reagents were from Sigma. Unlabeled lipids were obtained from Avanti Polar Lipids.
Cell Culture and Treatments.
Clone 9 rat hepatocytes were maintained in minimal essential medium (MEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in an atmosphere of 95% air/5% CO2. In a typical experiment, cells were plated onto coverslips (for immunofluorescence) or 35-mm dishes (for immunoperoxidase) and allowed to grow for 2 days before treatment with BFA and/or inhibitors as described (18). Specific details of each experiment will be given in the figure legends. Each inhibitor was freshly prepared as a 1000× stock solution in organic solvent (either 100% ethanol or dimethyl sulfoxide as appropriate) and then diluted into MEM just before use.
Immunocytochemistry.
Cells were processed for single or double immunofluorescence labeling (13) or for immunoperoxidase labeling and electron microscopy (EM) (26) as described. Primary antibodies used in these studies were as follows: polyclonal anti-α-mannosidase II (αManII) (from M. G. Farquhar, Univ. of California at San Diego, or K. Moreman, Univ. of Georgia); monoclonal anti-β-COP (from Affinity BioReagents); polyclonal anti-cation independent mannose 6-phosphate receptor (13); monoclonal anti-clathrin heavy chain (from Affinity BioReagents, Neshanic Station, NJ). Fluorescent secondary antibodies were from Jackson ImmunoResearch. Goat anti-rabbit IgG Fab fragments coupled to horseradish peroxidase were from Biosys (Compiegne, France).
In Vitro β-COP Binding.
Examination of the effects of BFA and PLA2 inhibitors on the association of β-COP with isolated Golgi membranes was performed essentially as described (16).
Lysophosphatidylcholine (lyso-PC) Analysis.
Cell-associated [3H]lyso-PC levels were measured from lipid extracts of clone 9 cells as described (27), except that lipids were resolved on aluminum-backed silica gel thin-layer chromatography plates (AL SIL G/UV; Whatman) (27). Spots corresponding to lyso-PC and PC were cut out and placed into liquid scintillation vials, and radioactivity was then measured by liquid scintillation counting. To determine the percent change in lyso-PC content, the ratio lyso-PC/(lyso-PC + PC) was calculated for each experimental condition, normalized to that obtained for the untreated control, and expressed as a percentage of that control. All values presented represent data garnered from duplicate experiments (all duplicate values differed by less than 10%).
RESULTS
In untreated clone 9 cells the Golgi complex appears as a juxtanuclear interconnected reticulum by immunofluorescence localization of the medial Golgi enzyme αManII (Fig. 1A). As expected, treatment with BFA resulted in the very rapid tubulation of Golgi membranes and their retrograde movement and fusion with the ER, generating, by 10 min, a characteristic diffuse staining pattern and nuclear envelope localization (11, 12)(Fig. 1B). Pretreatment of cells with the irreversible membrane-permeant PLA2 inhibitor BEL (28, 29), at 1 μM, slowed this BFA-induced process such that many cells contained Golgi-derived tubular intermediates (Fig. 1C). At 10 μM BEL the tubulation and retrograde transport of αManII were almost completely blocked (Fig. 1D). This inhibition of tubulation and retrograde transport was observed in several other cell types (HeLa, COS) and was confirmed by immuno-EM using anti-αManII antibodies (data not shown).
Figure 1.
Inhibition of BFA-stimulated Golgi membrane tubulation and reversible fragmentation of intact Golgi complexes by PLA2 inhibitors, as revealed by immunofluorescence of the resident Golgi enzyme αManII. (×720.) (A) Normal control cells. (B) Cells treated with BFA (5 μg/ml for 10 min). (C) Cells pretreated with the PLA2 inhibitor BEL (1 μM for 5 min) before addition of BFA (10 μg/ml) and continued incubation for another 10 min. (D) Cells treated as in C except BEL concentration was 10 μM. (E) Cells treated with the reversible PLA2 inhibitor ONO-RS-082 (ONO) (1 μM), for 30 min. (F) Cells treated with ONO-RS-082 as in E and then washed free of the drug and incubated for another 60 min in normal medium. All incubations were at 37°C.
Although PLA2 inhibitors blocked the tubulation and retrograde transport of Golgi membranes induced by BFA, immunofluorescence of αManII revealed that the Golgi complex was nevertheless altered from its normal morphology as it became “fragmented” under these conditions (Fig. 1D). In fact, we found that treatment of cells with low concentrations of PLA2 inhibitors alone, including the reversible inhibitor ONO-RS-082 (30), resulted in the formation of large Golgi fragments (Fig. 1E), which reassembled after removal of the drug (Fig. 1F). These large fragments always remained in the juxtanuclear region, did not diffuse into the peripheral cytoplasm, and were composed of cisternal stacks that appeared, by immunoperoxidase localization of αManII at the EM level, indistinguishable from typical Golgi stacks (Fig. 2). Similarly, we found that inhibition of BFA-stimulated Golgi membrane tubulation by ONO-RS-082 was reversible after washout of the inhibitor (data not shown).
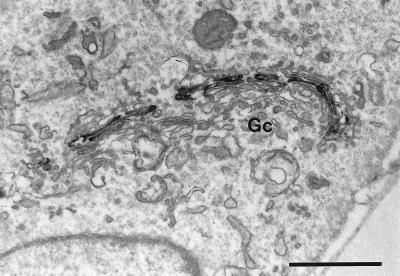
Figure 2.
Effect of the PLA2 inhibitor BEL on αManII localization as revealed by immunoperoxidase staining at the EM level. Cells treated with BEL alone (10 μM for 60 min), although fragmented as shown by immunofluorescence staining, retained typical stacked Golgi cisternae (Gc). (Bar = 1 μm.)
One possible explanation for the inhibitory effect of PLA2 antagonists is that they prevent the BFA-induced dissociation of COPI proteins from Golgi membranes, and hence, the tubulation and retrograde transport that otherwise ensue in the presence of BFA. However, double-immunofluorescence labeling with a monoclonal antibody against the cis Golgi antigen 10E6 (13) and polyclonal antibodies against β-COP revealed that treatment with ONO-RS-082 before BFA did not inhibit redistribution of β-COP from Golgi membranes to the cytosol (Fig. 3). Similarly, BEL did not inhibit BFA from causing the loss of β-COP from isolated Golgi membranes (Fig. 4). Thus, PLA2 inhibitors were not exerting their effects on Golgi-associated coat protein dynamics.
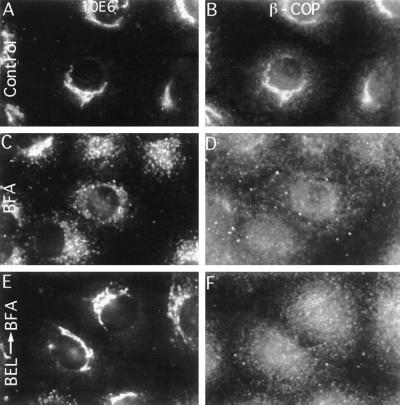
Figure 3.
PLA2 inhibitors do not prevent BFA-stimulated redistribution of β-COP from Golgi membranes as shown by double-immunofluorescence labeling with 10E6, a monoclonal antibody against a cis Golgi marker (Left) and polyclonal anti-β-COP antibodies (Right). (A and B) Control cells. (C and D) Cells treated with BFA alone (10 μg/ml for 10 min). (E and F) Cells pretreated with ONO-RS-082 (ONO) (10 μM for 5 min) before addition of BFA (10 μg/ml) and continued incubation for another 10 min. (×540.)
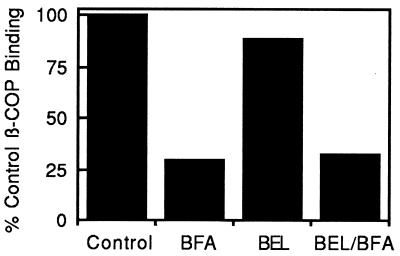
Figure 4.
BFA-induced loss of β-COP from isolated Golgi membranes in vitro occurs in the presence of the PLA2 inhibitor BEL. Isolated Golgi complexes were incubated with cytosol alone (Control), cytosol plus BFA (BFA), cytosol plus 25 μM BEL (BEL), or cytosol that was pretreated with BEL (25 μM) before addition of BFA (BEL/BFA). The amount of β-COP on pelleted and washed Golgi membranes was determined by Western blotting.
Another compartment that forms tubules in response to BFA is the TGN. In fact, at a given BFA concentration, TGN tubules form slightly slower than Golgi-derived tubules, and are therefore often more easily visualized by, for example, immunofluorescence localization of the cation-independent mannose 6-phosphate receptor (M6PR) (13). In control cells, M6PRs appeared in juxtanuclear tubules and vesicles of the TGN and endosomes (Fig. 5A), whereas treatment with BFA for 15 min resulted in the formation of extensive TGN tubules (Fig. 5C). Importantly, these tubules failed to form when cells were pretreated with ONO-RS-082 (10 μM) for just 5 min (Fig. 5E). As with β-COP on Golgi membranes, ONO-RS-082 prevented tubulation of the TGN but did not prevent the BFA-induced redistribution of clathrin complexes from TGN membranes to the cytosol (Fig. 5 B, D, and F). The inhibition of BFA-stimulated tubulation of TGN membranes by various PLA2 inhibitors was confirmed by immuno-EM using anti-M6PR antibodies (data not shown).
Figure 5.
PLA2 inhibitors prevent tubulation of TGN membranes, but not clathrin redistribution, as revealed by double-immunofluorescence imaging using polyclonal antibodies against the mannose 6-phosphate receptor (Left) and a monoclonal antibody against clathrin heavy chain (Right). (A and B) Control cells. (C and D) Cells treated with BFA alone (10 μg/ml for 10 min). (E and F) Cells pretreated with ONO-RS-082 (ONO) (10 μM for 5 min) before addition of BFA (10 μg/ml for 10 min). (×720.)
The ability to monitor the movement of Golgi and TGN proteins by immunofluorescence provided the basis for a sensitive quantitative assay of the effects of PLA2 inhibitors on BFA-stimulated tubulation. In BFA-treated cells, tubulation and retrograde transport of αManII to the ER was essentially complete by 5–10 min (Fig. 6). However, over the same time course, a variety of PLA2 inhibitors significantly slowed this process. Using this same assay with a fixed time point, we determined a dose–response and IC50 for various PLA2 inhibitors (Table 1). Significantly, all of the PLA2 inhibitors tested potently blocked BFA-stimulated tubulation of Golgi and TGN membranes (all at low micromolar concentrations). Among the more specific inhibitors of cytosolic PLA2s shown here to prevent BFA-stimulated tubulation are the site-specific reagents AACOCF3 and PACOCF3, trifluoromethyl ketone derivatives of arachidonic and palmitic acids, respectively (29, 31, 32), and BEL, as mentioned above, a mechanism-based suicide substrate that displays 1000-fold more selectivity for a cytosolic Ca2+-independent PLA2 vs. Ca2+-dependent PLA2 (28, 29).
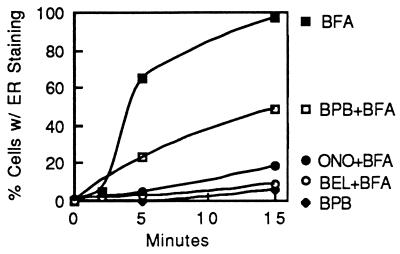
Figure 6.
Time course of BFA-induced retrograde movement of αManII from the Golgi complex to the ER and its inhibition by various PLA2 inhibitors. Cells were pretreated with various PLA2 inhibitors or solvent controls for 5 min, then BFA (10 μg/ml) was added and cells were incubated for up to 15 min. After treatment, cells were fixed and αManII was localized by immunofluorescence. Cells were then counted to determine the percentage in which αManII exhibited a diffuse, ER-like staining pattern that results from BFA-stimulated, tubule-mediated retrograde transport. BPB, bromophenacyl bromide; ONO, ONO-RS-082.
Table 1.
PLA2 inhibitors prevent BFA-stimulated Golgi recycling and TGN tubulation
| Compound | IC50, μM
|
|
|---|---|---|
| Golgi recycling | TGN tubulation | |
| PLA2 inhibitors | ||
| AACOCF3 | 6 | ND |
| ACA | 4 | ND |
| Aristolochic acid | 26 | ND |
| BEL | 4 | 20 |
| Bromophenacyl bromide | 5 | 12 |
| Dibucaine | 8 | 7 |
| ONO-RS-082 | 7 | 15 |
| PACOCF3 | 9 | ND |
| Quinacrine | 5 | 1 |
| Other inhibitors | ||
| Baicalein | NI | ND |
| Esculetin | NI | ND |
| ETI | NI | ND |
| Ibuprofen | NI | NI |
| Indomethacin | NI | NI |
| Neomycin sulfate | NI | NI |
| Prostaglandin E2 | NI | ND |
IC50 = the concentration at which 50% of cells were inhibited from exhibiting BFA-induced αManII staining in the ER (Golgi recycling) or tubulation of TGN membranes by mannose 6-phosphate receptor staining (TGN tubulation). The indicated numbers were obtained from visual inspection of dose–response curves for each inhibitor. ND, not done; NI, no inhibition to 50% at 100 μM.
Tubulation of both Golgi and TGN membranes in vivo is greatly facilitated by microtubules (11–13), so we examined cells treated with PLA2 inhibitors by immunofluorescence with anti-tubulin antibodies. We found no obvious effects on microtubule number or distribution, except in the case of bromophenacyl bromide (BPB) which, on its own, significantly depolymerized microtubules (data not shown). However, the effect of BPB on microtubules could be separated from its inhibitory effect on tubulation by first stabilizing cytoplasmic microtubules with Taxol (data not shown).
An intracellular PLA2, catalyzing the hydrolysis of glycerophospholipids into lysophospholipids and free fatty acids, could induce tubulation by directly altering membrane structure (33, 34), or alternatively, the downstream metabolites of phospholipid hydrolysis could act indirectly as signaling molecules to somehow regulate tubulation. The best-studied intracellular PLA2 product is arachidonic acid, which can be enzymatically converted to numerous bioactive signaling molecules via the cyclooxygenase, lipoxygenase, and cytochrome P450 pathways (35). Thus, blocking the subsequent metabolism of arachidonic acid might also affect tubulation. However, using a variety of specific and nonspecific inhibitors of arachidonic acid metabolism, including indomethacin, ibuprofen, esculetin, baicalein, and ETI (Table 1), we found no effect on BFA-stimulated tubulation. Similarly, we found that neomycin sulfate, an inhibitor of phospholipases C and D at the concentrations tested here (36), had no inhibitory effects.
Finally, to demonstrate that the PLA2 antagonists were in fact inhibiting the activities of these enzymes, we measured in cell extracts lyso-PC levels, which would be expected to decrease in the presence of PLA2 inhibitors. Using conditions of BEL treatment previously shown decrease lyso-PC levels in other cell types (27), we found that treatment of clone 9 cells with BEL reduced the amount of lyso-PC in cell extracts to 71% of control levels, a reduction roughly comparable to that seen in other cells (27). Importantly, a similar reduction (to 69% of controls) was seen when BFA (10 μg/ml) was added in the presence of BEL, suggesting that BFA did not perturb this compound’s ability to inhibit the relevant phospholipase activities.
DISCUSSION
The idea that a cytosolic tubulation factor, operating at the level of the Golgi complex, might work enzymatically was suggested from our in vitro studies in which isolated Golgi complexes were induced to form membrane tubules by using very low concentrations a heat-labile fraction of cytosolic proteins that is highly enriched in tubulation activity (16, 17). Our finding here that a wide variety of both general and specific PLA2 inhibitors potently block Golgi and TGN membrane tubulation is consistent with these previous studies. The effect of the PLA2 inhibitors was rapid (within 5 min of addition), suggesting that they were influencing events closely or directly related to the formation of Golgi tubules.
Although these in vivo studies cannot definitively identify the type of phospholipase activity that participates in BFA-stimulated tubulation, they are consistent with the idea that cytoplasmic PLA2 activity participates in this process. For example, the inhibitors aristolochic acid, ACA, and ONO-RS-082, as well as the more specific substrate analogs AACOCF3 and PACOCF3, are specific and potent inhibitors of cytoplasmic PLA2s (29, 31). Moreover, the IC50 values of these inhibitors on tubulation were generally very close to those for inhibition of known PLA2 activities (24). Our results here showing that tubulation is potently inhibited by BEL are particularly interesting in light of recent studies showing that this inhibitor is 1000-fold more selective for a Ca2+-independent PLA2 than for its Ca2+-dependent counterpart (28, 29). Moreover, this ≈85-kDa Ca2+-independent PLA2 interacts with calmodulin (23), an intriguing finding in light of our previous studies showing that membrane tubulation of multiple organelles in vivo is sensitive to calmodulin antagonists (18). However, because some of the examined compounds may inhibit enzymes that are also known to exhibit phospholipase A1, platelet-activating-factor hydrolase, and lysophospholipase activities in various in vitro and in vivo systems (22), we cannot rule out the possibility that these kinds of enzyme activities may also contribute to the tubulation events. Preliminary studies utilizing our cytosol-dependent in vitro tubulation assay show that these same PLA2 inhibitors potently block tubule formation (P.d.F., R.S., and W.J.B., unpublished data), indicating that this assay should be useful for identifying the exact phospholipase activity responsible for Golgi membrane tubulation.
The mechanism by which a PLA2 could impart such a dramatic change in membrane shape in a biological setting is unclear. One possibility is that downstream metabolites of PLA2 hydrolysis, such as those generated during arachidonic acid metabolism, could serve as signaling molecules to stimulate indirectly the tubulation machinery. Though our studies showing that a variety of inhibitors of arachidonic acid metabolism had no effect on BFA-stimulated tubulation of Golgi membranes argue against this idea, we cannot unequivocally rule out this possibility because the relevant pathways may not have been targeted, or sufficiently inhibited.
Another possibility, not mutually exclusive of any signaling role, is that hydrolysis of a phospholipid into a lysophospholipid and free fatty acid is directly responsible for inducing tubule growth. Could such a mechanism produce this kind of dramatic membrane transformation? In fact, Sheetz and Singer (37) proposed the bilayer couple hypothesis to account for this type of membrane behavior. This model articulates the idea that the two leaflets of a phospholipid bilayer are inexorably coupled such that a change in the surface area of one leaflet forces both leaflets of the membrane bilayer to accommodate by bending. For example, increasing the surface area in the outer leaflet of a red blood cell (33, 37) or artificial liposome (34) results in the formation of blebs or tubules that extend out from the membrane bilayer. Could a PLA2 generate such a change in surface area to produce tubules? Biophysical studies have established that hydrolysis by PLA2 of glycerophospholipids (e.g., PC) within membranes can produce dramatic shape changes, including the formation of blebs and tubules (33, 38). Moreover, inserting lysophospholipids into the outer leaflet of artificial liposomes can result in the formation of membrane tubules (34), which are remarkably similar in size and morphology to Golgi and TGN-derived tubules. Thus, the suggestion that tubulation of Golgi and TGN membranes in vivo may depend on a cytosolic PLA2 activity that liberates lysophospholipids and fatty acids, thus increasing local lysophospholipid concentrations, is consistent with biophysical studies on membrane shape changes. In this regard, we could not measure a detectable increase in lyso-PC levels in extracts from BFA-treated cells. However, any such increase may be difficult to detect, given that only a small fraction of intracellular membranes tubulate in response to BFA, and that only a small change in the mole fraction of lipids within a half bilayer is required to impart large shape changes (33, 37). Of course, the generation of phospholipid metabolites might have other effects that could contribute to changes in membrane structure, including generation of lipid microdomains, phase changes, or the selective association of membrane or peripheral proteins.
Several studies have shown that membrane tubule formation from the Golgi complex and TGN is greatly facilitated by, but not absolutely dependent upon, microtubules (10–16). On the basis of these observations, and our results here, we speculate that Golgi membrane tubulation is at least a two-step process involving, first, the phospholipase-dependent formation of short membrane tubules, which, secondarily, are captured and pulled along microtubules by kinesin motor proteins (15) to facilitate tubule-mediated trafficking events.
The exact role that Golgi-derived tubules play in membrane trafficking is unclear. Their presence and possible contribution to Golgi-to-ER retrograde trafficking is most evident in BFA-treated cells; however, the extent to which they contribute to this pathway in untreated cells is unknown. Direct imaging of live cells by using chimeras consisting of Golgi resident enzymes fused with green fluorescent protein indicate that tubules are continuously formed from Golgi membranes (14). Although the exact fate of these tubules (recycling to the ER?) has not been unequivocally demonstrated, these studies are consistent with the idea that Golgi-derived tubules play a role in retrograde transport. We have, in fact, recently found that normal Golgi-to-ER retrograde trafficking, as assayed by visualizing a variety of proteins that bidirectionally traffic between the ER and Golgi complex, is potently inhibited by the same PLA2 inhibitors shown here to block BFA-stimulated Golgi tubulation and recycling (unpublished data).
Another possible functional role for membrane tubules may be to link separate cisternal Golgi stacks into a large interconnected organelle (39). Numerous studies using, for example, three-dimensional image reconstructions from serial thin sections and fluorescence imaging of Golgi morphology have documented the existence of such tubular interconnections (4–8). Our finding that PLA2 inhibitors on their own caused the Golgi complex to separate into large fragments, which retained the typical Golgi stacked cisternal architecture, suggests that maintenance of this architecture may require a dynamic process of PLA2-dependent tubule formation. The fragmented Golgi stacks produced by PLA2 inhibitors are quite different from those generated after depolymerization of microtubules, which in the later case appear to form because nascent Golgi stacks, assembling from recycled Golgi resident proteins at peripheral ER export sites, fail to converge at the microtubule organizing center (40). Therefore, studies with PLA2 inhibitors, at the very least, point to the possibility that membrane tubules may serve as trafficking intermediates in several Golgi-associated processes.
Acknowledgments
This work was supported by National Institutes of Health Grant DK51596 to W.J.B.
ABBREVIATIONS
- AACOCF3
arachidonyl trifluoromethyl ketone
- ACA
N-(p-amylcinnamoyl)anthranilic acid
- acid
BEL, bromoenol lactone
- BFA
brefeldin A
- ER
endoplasmic reticulum
- EM
electron microscopy
- ETI
eicosatriynoic acid
- αManII
α-mannosidase II
- ONO-RS-082
2-(p-amylcinnamoyl)amino-4-cholorobenzoic acid
- PACOCF3
palmitoyl trifluoromethyl ketone
- PC
phosphatidylcholine
- PLA2
phospholipase A2
- TGN
trans Golgi network
References
- 1.Farquhar M G, Palade G E. Trends Cell Biol. 1998;8:2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 3.Schekman R, Orci L. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 4.Mollenhauer H H, Morré D J. J Cell Biol. 1966;29:373–376. doi: 10.1083/jcb.29.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novikoff P M, Novikoff A B, Quintana N, Hauw J-J. J Cell Biol. 1971;50:859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rambourg A, Clermont Y, Hermo L. J Anat. 1979;154:455–476. doi: 10.1002/aja.1001540402. [DOI] [PubMed] [Google Scholar]
- 7.Rambourg A, Clermont Y. Eur J Cell Biol. 1990;51:189–200. [PubMed] [Google Scholar]
- 8.Cooper M S, Cornell-Bell A H, Chernjavsky A, Dani J W, Smith S J. Cell. 1990;61:135–145. doi: 10.1016/0092-8674(90)90221-y. [DOI] [PubMed] [Google Scholar]
- 9.Weidman P, Roth R, Heuser J. Cell. 1993;75:123–133. [PubMed] [Google Scholar]
- 10.Klausner R D, Donaldson J G, Lippincott-Schwartz J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippincott-Schwartz J, Donaldson J G, Schweizer A, Berger E G, Hauri H-P, Yuan L C, Klausner R D. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 12.Lippincott-Schwartz J, Yuan L C, Tipper C, Amherdt M, Orci L, Klausner R D. Cell. 1991;67:601–617. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 13.Wood S A, Park J E, Brown W J. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- 14.Sciaky N, Presley J, Smith C, Zaal K J M, Cole N, Moreira J E, Terasaki M, Siggia E, Lippincott-Schwartz J. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippincott-Schwartz J, Cole N B, Marotta A, Conrad P A, Bloom G S. J Cell Biol. 1995;128:293–306. doi: 10.1083/jcb.128.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cluett E B, Wood S A, Banta M, Brown W J. J Cell Biol. 1993;120:15–24. doi: 10.1083/jcb.120.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banta M, Polizotto R S, Wood S A, de Figueiredo P, Brown W J. Biochemistry. 1995;34:13359–13366. doi: 10.1021/bi00041a012. [DOI] [PubMed] [Google Scholar]
- 18.de Figueiredo P, Brown W J. Mol Biol Cell. 1995;6:871–887. doi: 10.1091/mbc.6.7.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apodaca G, Enrich C, Mostov K E. J Biol Chem. 1994;269:19005–10913. [PubMed] [Google Scholar]
- 20.Klee C B. In: Molecular Aspects of Cellular Regulation. Cohen P, Klee C B, editors. New York: Elsevier; 1988. pp. 35–56. [Google Scholar]
- 21.Dennis E A. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 22.Mukherjee A B, Miele L, Pattabiraman N. Biochem Pharmacol. 1994;48:1–10. doi: 10.1016/0006-2952(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 23.Wolf M J, Gross R W. J Biol Chem. 1996;271:30879–30885. doi: 10.1074/jbc.271.48.30879. [DOI] [PubMed] [Google Scholar]
- 24.Gelb M H, Jain M K, Berg O G. FASEB J. 1994;8:917–924. doi: 10.1096/fasebj.8.12.8088457. [DOI] [PubMed] [Google Scholar]
- 25.Daniels S B, Cooney E, Sofia M J, Chakravarty P K, Katzenellenbogen J A. J Biol Chem. 1983;258:15046–15053. [PubMed] [Google Scholar]
- 26.Brown W J, Farquhar M G. Methods Cell Biol. 1989;31:553–569. doi: 10.1016/s0091-679x(08)61626-x. [DOI] [PubMed] [Google Scholar]
- 27.Balsinde J, Bianco I D, Ackermann E J, Conde-Frieboes K, Dennis E A. Proc Natl Acad Sci USA. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazen S L, Zupan L A, Weiss R H, Getman D P, Gross R W. J Biol Chem. 1991;266:7227–7232. [PubMed] [Google Scholar]
- 29.Ackermann E J, Conde-Frieboes K, Dennis E A. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- 30.Banga H S, Simons E R, Brass L F, Rittenhouse S E. Proc Natl Acad Sci USA. 1986;83:9197–9201. doi: 10.1073/pnas.83.23.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Street I P, Lin H K, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay N M, Huang Z, Weech P K, Gelb M H. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 32.Trimble L A, Street I P, Perrier H, Tremblay N M, Weech P K, Bernstein M A. Biochemistry. 1993;32:12560–12565. doi: 10.1021/bi00210a002. [DOI] [PubMed] [Google Scholar]
- 33.Christiansson A, Kuyprs F A, Roelofsen B, Op Den Kamp J A F, van Deenen L L M. J Cell Biol. 1985;101:1455–1462. doi: 10.1083/jcb.101.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mui B L-S, Dobereiner H-G, Madden T D, Cullis P R. Biophys J. 1995;69:930–941. doi: 10.1016/S0006-3495(95)79967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Needleman P, Turk J, Jakschik B A, Morrison A R, Lefkowith J B. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 36.Liscovitch M, Chalifa V, Danin M, Eli V. Biochem J. 1991;279:319–322. doi: 10.1042/bj2790319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheetz M P, Singer S J. Proc Natl Acad Sci USA. 1974;71:4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Israelachvili J N, Marcelja S, Horn R G. Q Rev Biophys. 1982;13:121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- 39.Lucocq J M, Warren G. EMBO J. 1987;6:3239–3246. doi: 10.1002/j.1460-2075.1987.tb02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole N B, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Mol Biol Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]