Abstract
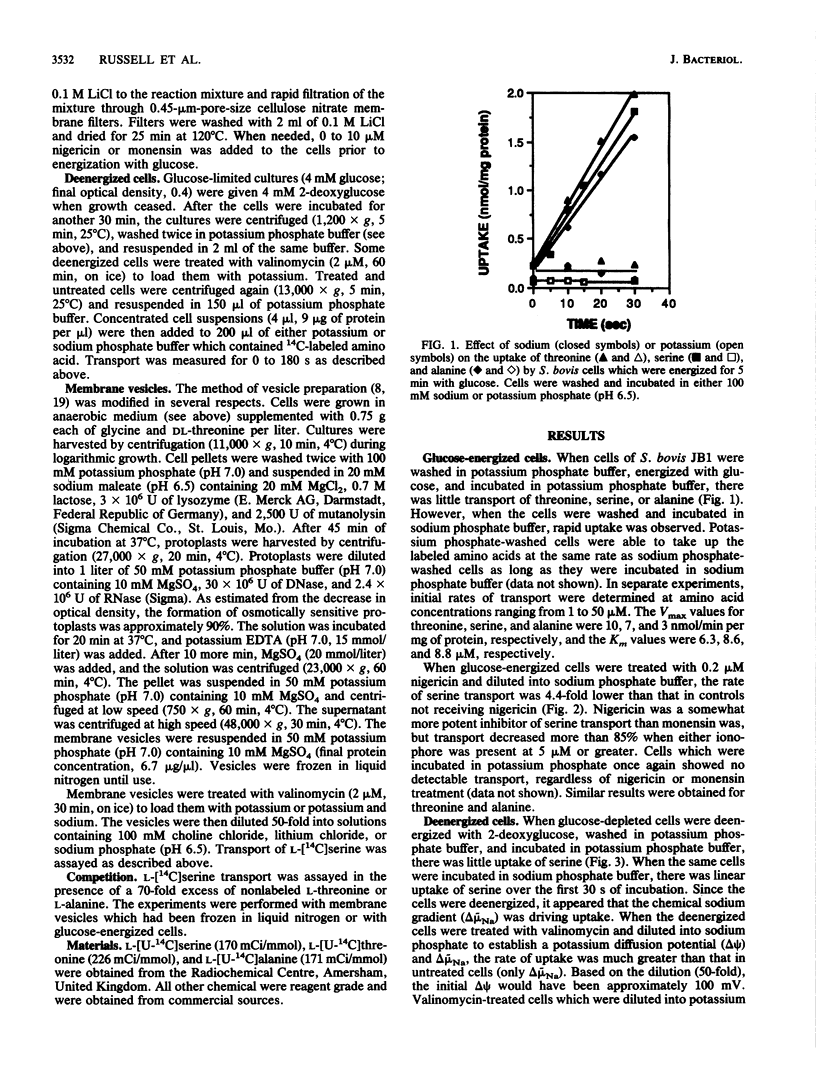
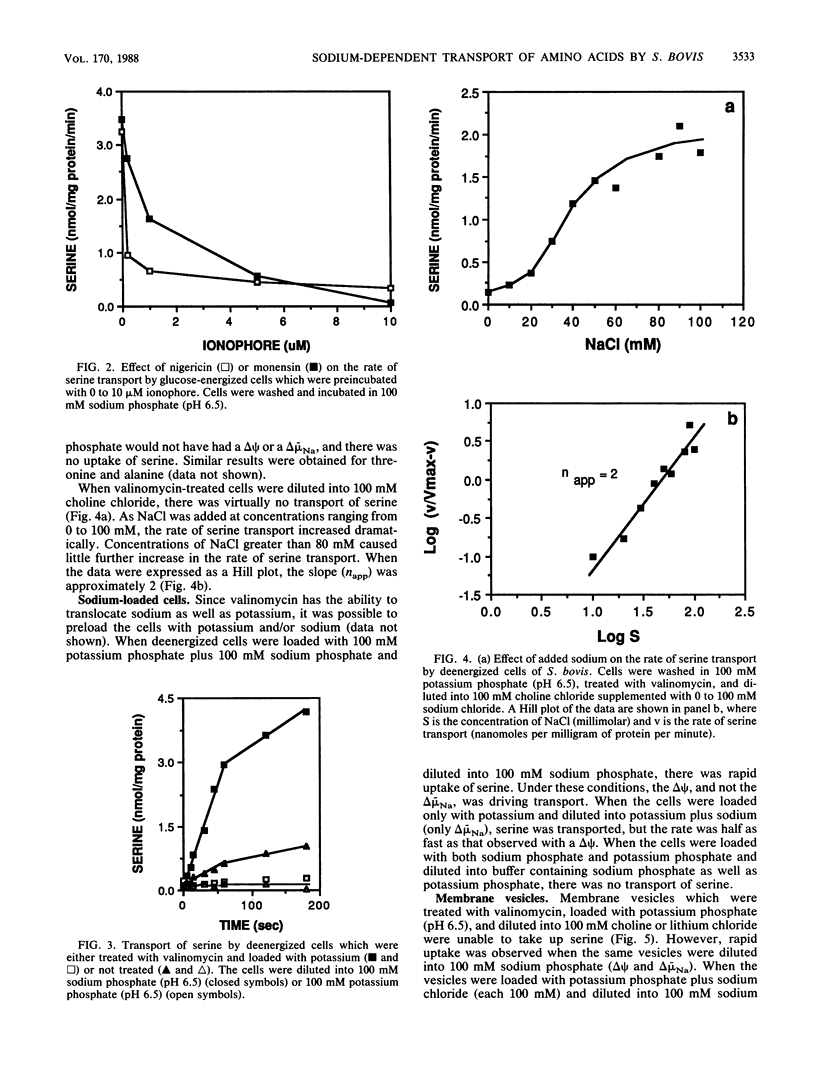
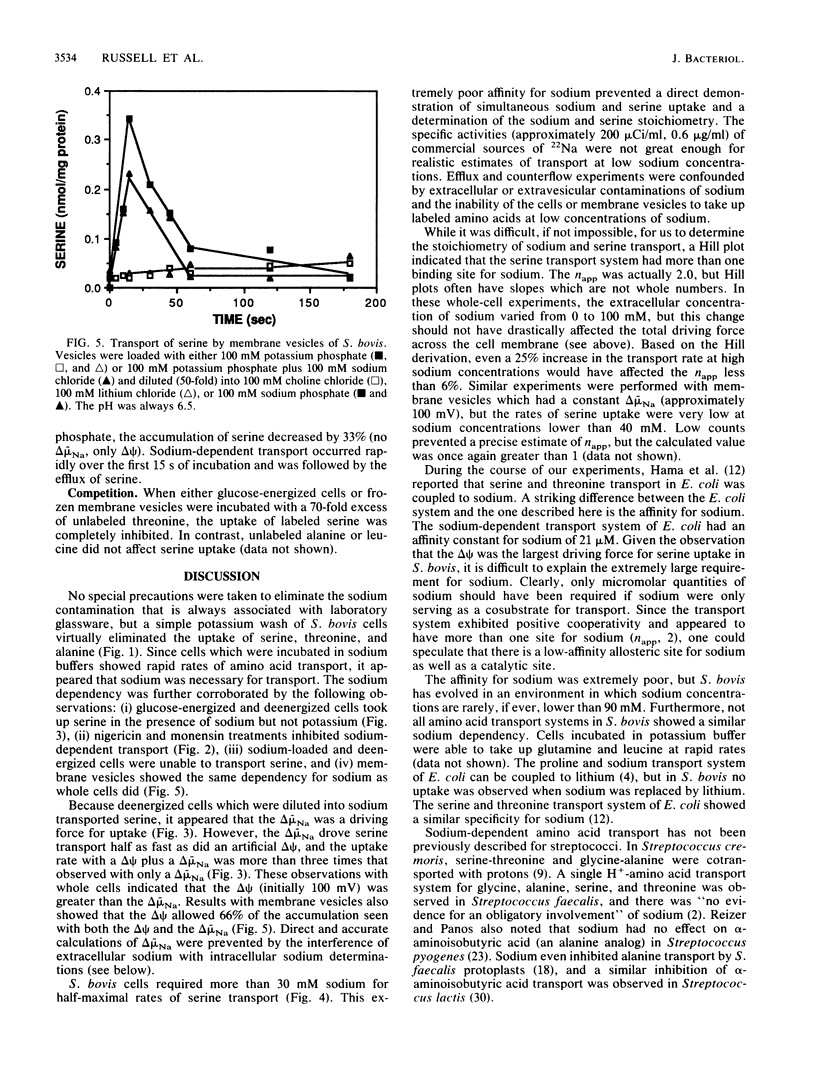
Streptococcus bovis JB1 cells were able to transport serine, threonine, or alanine, but only when they were incubated in sodium buffers. If glucose-energized cells were washed in potassium phosphate and suspended in potassium phosphate buffer, there was no detectable uptake. Cells deenergized with 2-deoxyglucose and incubated in sodium phosphate buffer were still able to transport serine, and this result indicated that the chemical sodium gradient was capable of driving transport. However, when the deenergized cells were treated with valinomycin and diluted into sodium phosphate to create both an artificial membrane potential and a chemical sodium gradient, rates of serine uptake were fivefold greater than in cells having only a sodium gradient. If deenergized cells were preloaded with sodium (no membrane potential or sodium gradient), there was little serine transport. Nigericin and monensin, ionophores capable of reversing sodium gradients across membranes, strongly inhibited sodium-dependent uptake of the three amino acids. Membrane vesicles loaded with potassium and diluted into either lithium or choline chloride were unable to transport serine, but rapid uptake was evident if sodium chloride was added to the assay mixture. Serine transport had an extremely poor affinity for sodium, and more than 30 mM was needed for half-maximal rates of uptake. Serine transport was inhibited by an excess of threonine, but an excess of alanine had little effect. Results indicated that S. bovis had separate sodium symport systems for serine or threonine and alanine, and either the membrane potential or chemical sodium gradient could drive uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Caldwell D. R., Hudson R. F. Sodium, an obligate growth requirement for predominant rumen bacteria. Appl Microbiol. 1974 Mar;27(3):549–552. doi: 10.1128/am.27.3.549-552.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Tsuchiya T., Yamane Y., Wood J. M., Wilson T. H. Na+ (Li+)-proline cotransport in Escherichia coli. J Membr Biol. 1985;84(2):157–164. doi: 10.1007/BF01872213. [DOI] [PubMed] [Google Scholar]
- Chen G., Strobel H. J., Russell J. B., Sniffen C. J. Effect of hydrophobicity of utilization of peptides by ruminal bacteria in vitro. Appl Environ Microbiol. 1987 Sep;53(9):2021–2025. doi: 10.1128/aem.53.9.2021-2025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donius D. A., Simpson M. E., Marsh P. B. Effect of monensin fed with forage on digestion and the ruminal ecosystem of steers. J Anim Sci. 1976 Jan;42(1):229–234. doi: 10.2527/jas1976.421229x. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Jong S., Konings W. N. Transport of branched-chain amino acids in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1987 Nov;169(11):5193–5200. doi: 10.1128/jb.169.11.5193-5200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droniuk R., Wong P. T., Wisse G., Macleod R. A. Variation in Quantitative Requirements for Na for Transport of Metabolizable Compounds by the Marine Bacteria Alteromonas haloplanktis 214 and Vibrio fischeri. Appl Environ Microbiol. 1987 Jul;53(7):1487–1495. doi: 10.1128/aem.53.7.1487-1495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklund C. V., Glass T. L. Glucose uptake by the cellulolytic ruminal anaerobe Bacteroides succinogenes. J Bacteriol. 1987 Feb;169(2):500–506. doi: 10.1128/jb.169.2.500-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E., DOUGHERTY R. W., BRYANT M. P., CELLO R. M. Microbiological and physiological changes associated with acute indigestion in sheep. Cornell Vet. 1952 Oct;42(4):423–449. [PubMed] [Google Scholar]
- Hama H., Shimamoto T., Tsuda M., Tsuchiya T. Properties of a Na+-coupled serine-threonine transport system in Escherichia coli. Biochim Biophys Acta. 1987 Dec 11;905(2):231–239. doi: 10.1016/0005-2736(87)90451-2. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hardie M. M. Periplasmic protein associated with the oligopeptide permeases of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Sep;155(3):1434–1438. doi: 10.1128/jb.155.3.1434-1438.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A. Bioenergetics of alkalophilic bacteria. J Membr Biol. 1986;89(2):113–125. doi: 10.1007/BF01869707. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1987 Oct;53(10):2388–2393. doi: 10.1128/aem.53.10.2388-2393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne J. W. Transport and hydrolysis of peptides by microorganisms. Ciba Found Symp. 1977;(50):305–334. doi: 10.1002/9780470720318.ch17. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- ROGOSA M., FRANKLIN J. G., PERRY K. D. Correlation of the vitamin requirements with cultural and biochemical characters of Lactobacillus spp. J Gen Microbiol. 1961 Jul;25:473–482. doi: 10.1099/00221287-25-3-473. [DOI] [PubMed] [Google Scholar]
- Reizer J., Panos C. Transport of alpha-aminoisobutyric acid by Streptococcus pyogenes and its derived L-form. J Bacteriol. 1982 Jan;149(1):211–220. doi: 10.1128/jb.149.1.211-220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. A proposed mechanism of monensin action in inhibiting ruminal bacterial growth: effects on ion flux and protonmotive force. J Anim Sci. 1987 May;64(5):1519–1525. doi: 10.2527/jas1987.6451519x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Dombrowski D. B. Effect of pH on the efficiency of growth by pure cultures of rumen bacteria in continuous culture. Appl Environ Microbiol. 1980 Mar;39(3):604–610. doi: 10.1128/aem.39.3.604-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Robinson P. H. Compositions and characteristics of strains of Streptococcus bovis. J Dairy Sci. 1984 Jul;67(7):1525–1531. doi: 10.3168/jds.S0022-0302(84)81471-X. [DOI] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Hasan S. M., Raven J. Glutamate transport driven by an electrochemical gradient of sodium ions in Escherichia coli. J Bacteriol. 1977 Sep;131(3):848–853. doi: 10.1128/jb.131.3.848-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J., MANNING G. B., NELSON W. O. Ammonium salts as a sole source of nitrogen for the growth of Streptococcus bovis. J Bacteriol. 1959 Jul;78(1):147–147. doi: 10.1128/jb.78.1.147-147.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. E., Hungate R. E. Amino acid concentrations in rumen fluid. Appl Microbiol. 1967 Jan;15(1):148–151. doi: 10.1128/am.15.1.148-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boven A., Konings W. N. A Phosphate-Bond-Driven Dipeptide Transport System in Streptococcus cremoris Is Regulated by the Internal pH. Appl Environ Microbiol. 1987 Dec;53(12):2897–2902. doi: 10.1128/aem.53.12.2897-2902.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


