Abstract
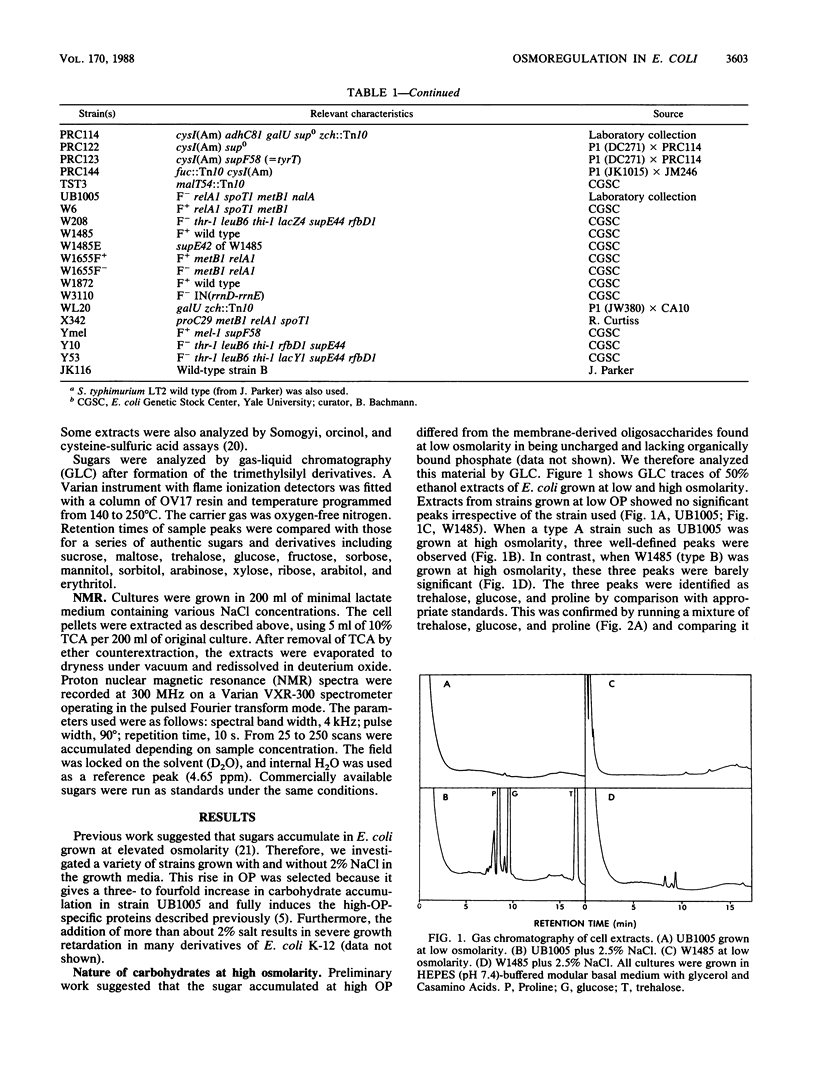
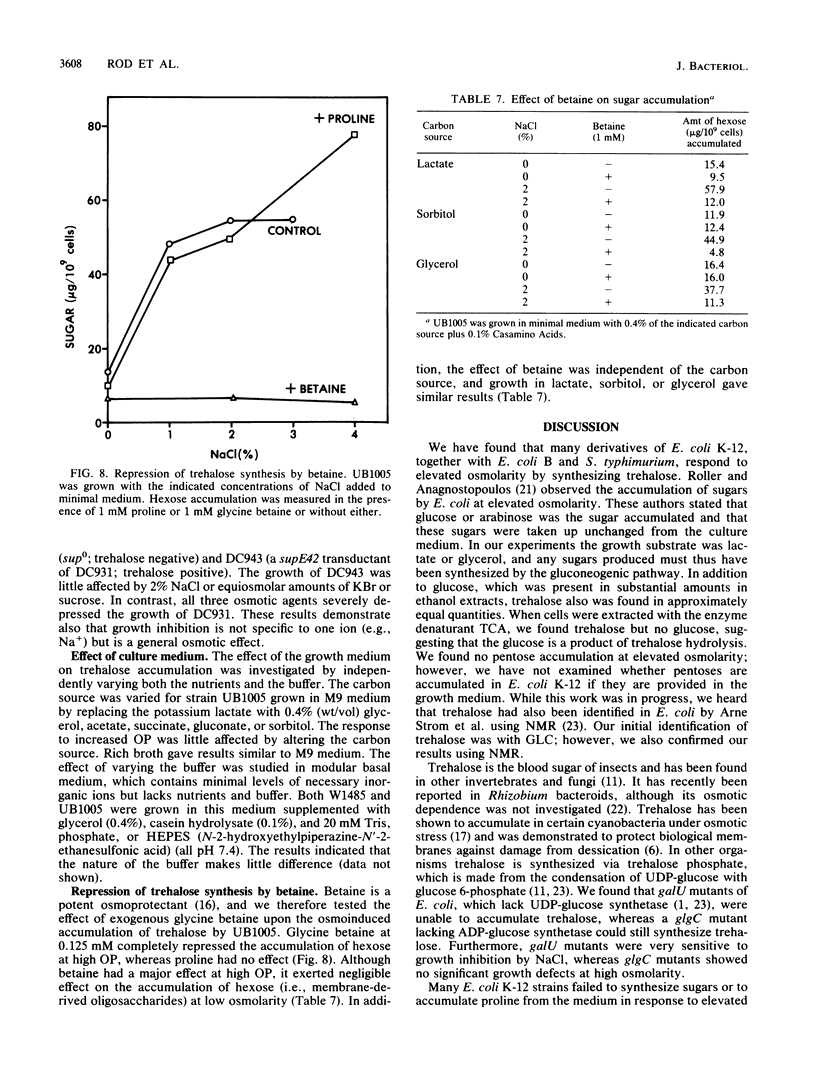
When grown at high osmotic pressure, some strains of Escherichia coli K-12 synthesized substantial levels of free sugar and accumulated proline if it was present in the growth medium. The sugar was identified as trehalose by chemical reactivity, gas-liquid chromatography, and nuclear magnetic resonance spectroscopy. Strains of E. coli K-12 could be divided into two major classes with respect to osmoregulation. Those of class A showed a large increase in trehalose levels with increasing medium osmolarity and also accumulated proline from the medium, whereas those in class B showed no accumulation of trehalose or proline. Most class A strains carried suppressor mutations which arose during their derivation from the wild type, whereas the osmodefective strains of class B were suppressor free. When amber suppressor mutations at the supD, supE, or supF loci were introduced into such sup0 osmodefective strains, they became osmotolerant and gained the ability to accumulate trehalose in response to elevated medium osmolarity. It appears that the original K-12 strain of E. coli carries an amber mutation in a gene affecting osmoregulation. Mutants lacking ADP-glucose synthetase (glgC) accumulated trehalose normally, whereas mutants lacking UDP-glucose synthetase (galU) did not make trehalose and grew poorly in medium of high osmolarity. Trehalose synthesis was repressed by exogenous glycine betaine but not by proline.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. D. Microbial water stress. Bacteriol Rev. 1976 Dec;40(4):803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. P. Mutant of Escherichia coli deficient in osmoregulation of periplasmic oligosaccharide synthesis. J Bacteriol. 1985 Mar;161(3):1049–1053. doi: 10.1128/jb.161.3.1049-1053.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. A third L-proline permease in Salmonella typhimurium which functions in media of elevated osmotic strength. J Bacteriol. 1982 Sep;151(3):1433–1443. doi: 10.1128/jb.151.3.1433-1443.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182(1):82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- De Felice M., Squires C., Levinthal M., Guardiola J., Lamberti A., Iaccarino M. Growth inhibition of Escherichia coli K-12 by L-valine: a consequence of a regulatory pattern. Mol Gen Genet. 1977 Nov 4;156(1):1–7. doi: 10.1007/BF00272245. [DOI] [PubMed] [Google Scholar]
- Dunlap V. J., Csonka L. N. Osmotic regulation of L-proline transport in Salmonella typhimurium. J Bacteriol. 1985 Jul;163(1):296–304. doi: 10.1128/jb.163.1.296-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. The metabolism of alpha,alpha-trehalose. Adv Carbohydr Chem Biochem. 1974;30:227–256. doi: 10.1016/s0065-2318(08)60266-8. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Roller S. D., Anagnostopoulos G. D. Accumulation of carbohydrate by Escherichia coli B/r/1 during growth at low water activity. J Appl Bacteriol. 1982 Jun;52(3):425–434. doi: 10.1111/j.1365-2672.1982.tb05073.x. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Accumulation of alpha,alpha-trehalose by Rhizobium bacteria and bacteroids. J Bacteriol. 1985 Oct;164(1):78–84. doi: 10.1128/jb.164.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



