Abstract
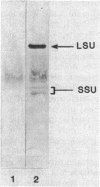
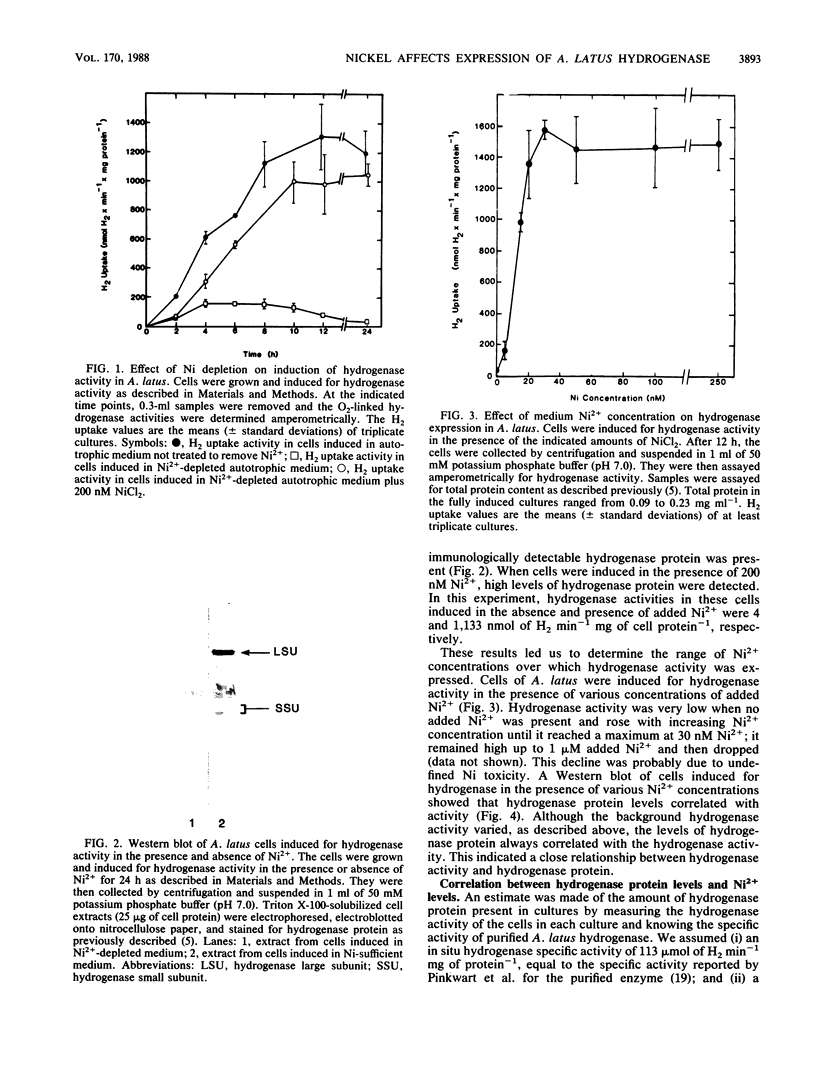
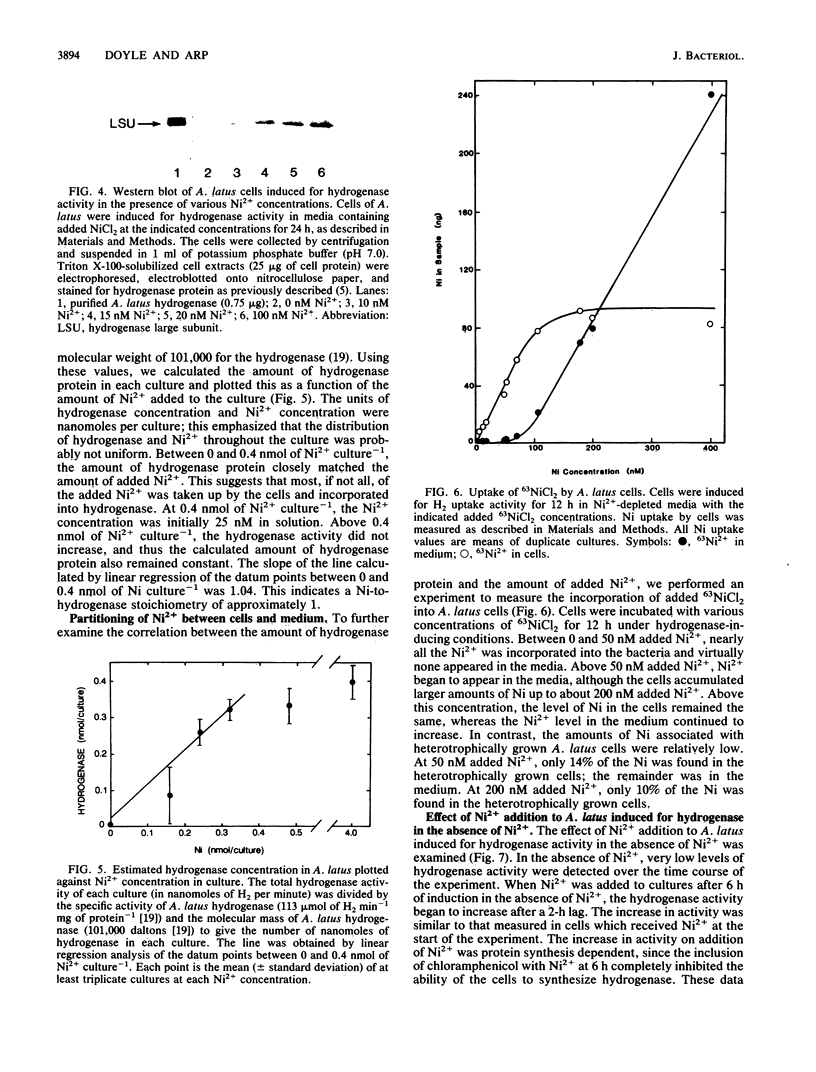
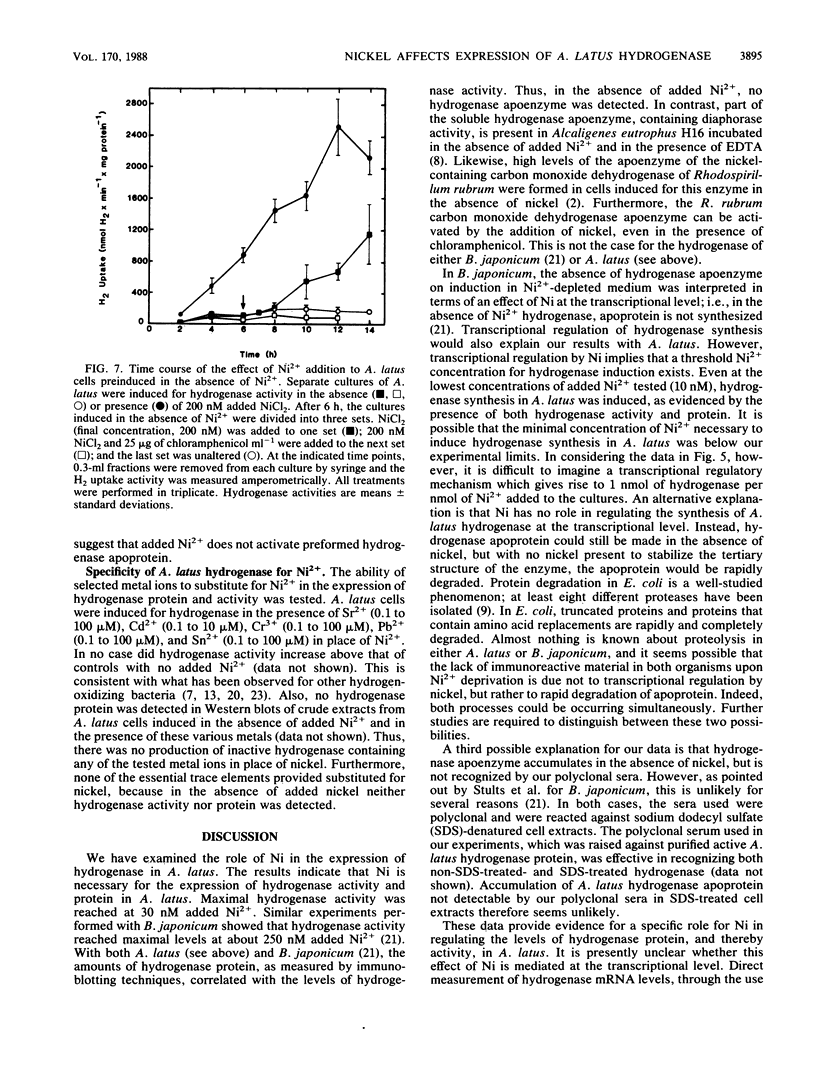
The effects of nickel on the expression of hydrogenase in the hydrogen-oxidizing bacterium Alcaligenes latus were studied. In the absence of added nickel, both hydrogenase activity, measured as O2-dependent H2 uptake, and hydrogenase protein, measured in a Western immunoblot, were very low compared with the levels in cells induced for hydrogenase in the presence of nickel. Hydrogenase activity and protein levels were dependent on the added nickel concentration and were saturated at 30 nM added Ni2+. The amount of hydrogenase protein in a culture at a given nickel concentration was calculated from the H2 uptake activity of the culture at that Ni2+ concentration. Between 0 and 30 nM added Ni2+, the amount of hydrogenase protein (in nanomoles) was stoichiometric with the amount of added Ni2+. Thus, all of the added Ni2+ could be accounted for in hydrogenase. Between 0 and 50 nM added Ni2+, all the Ni present in the cultures was associated with the cells after 12 h; above 50 nM added Ni2+, some Ni remained in the medium. No other divalent metal cations tested were able to substitute for Ni2+ in the formation of active hydrogenase. We suggest two possible mechanisms for the regulation of hydrogenase activity and protein levels by nickel.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonam D., McKenna M. C., Stephens P. J., Ludden P. W. Nickel-deficient carbon monoxide dehydrogenase from Rhodospirillum rubrum: in vivo and in vitro activation by exogenous nickel. Proc Natl Acad Sci U S A. 1988 Jan;85(1):31–35. doi: 10.1073/pnas.85.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R., Patil D. S., Fernandez V. M. Electron-spin-resonance/electron-paramagnetic-resonance spectroscopy of iron-sulphur enzymes. Biochem Soc Trans. 1985 Jun;13(3):572–578. doi: 10.1042/bst0130572. [DOI] [PubMed] [Google Scholar]
- Doyle C. M., Arp D. J. Regulation of H2 oxidation activity and hydrogenase protein levels by H2, O2, and carbon substrates in Alcaligenes latus. J Bacteriol. 1987 Oct;169(10):4463–4468. doi: 10.1128/jb.169.10.4463-4468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew D. L., Welch R. M., Cary E. E. A simple plant nutrient solution purification method for effective removal of trace metals using controlled pore glass-8-hydroxyquinoline chelation column chromatography. Plant Physiol. 1984 Sep;76(1):103–105. doi: 10.1104/pp.76.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Premakumar R., Bishop P. E. Transcriptional regulation of nitrogen fixation by molybdenum in Azotobacter vinelandii. J Bacteriol. 1986 Aug;167(2):480–486. doi: 10.1128/jb.167.2.480-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucas R. V., Hanus F. J., Russell S. A., Evans H. J. Nickel: A micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2253–2257. doi: 10.1073/pnas.80.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Fox J. A., Hausinger R. P., Daniels L., Orme-Johnson W. H., Walsh C. Paramagnetic centers in the nickel-containing, deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1983 Jan;80(2):378–382. doi: 10.1073/pnas.80.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGall J., Ljungdahl P. O., Moura I., Peck H. D., Jr, Xavier A. V., Moura J. J., Teixera M., Huynh B. H., DerVartanian D. V. The presence of redox-sensitive nickel in the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1982 May 31;106(2):610–616. doi: 10.1016/0006-291x(82)91154-8. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Pinkwart M., Schneider K., Schlegel H. G. Purification and properties of the membrane-bound hydrogenase from N2-fixing Alcaligenes latus. Biochim Biophys Acta. 1983 Jun 29;745(3):267–278. doi: 10.1016/0167-4838(83)90058-4. [DOI] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Organization, expression, and evolution of genes for mercury resistance. Annu Rev Microbiol. 1986;40:607–634. doi: 10.1146/annurev.mi.40.100186.003135. [DOI] [PubMed] [Google Scholar]
- Teixeira M., Moura I., Xavier A. V., Huynh B. H., DerVartanian D. V., Peck H. D., Jr, LeGall J., Moura J. J. Electron paramagnetic resonance studies on the mechanism of activation and the catalytic cycle of the nickel-containing hydrogenase from Desulfovibrio gigas. J Biol Chem. 1985 Jul 25;260(15):8942–8950. [PubMed] [Google Scholar]
- Veillon C., Vallee B. L. Atomic spectroscopy in metal analysis of enzymes and other biological material. Methods Enzymol. 1978;54:446–484. doi: 10.1016/s0076-6879(78)54028-7. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Beier A., Pecher A., Wirth R., Böck A. Regulation of the synthesis of hydrogenase (formate hydrogen-lyase linked) of E. coli. Arch Microbiol. 1984 Nov;139(4):299–304. doi: 10.1007/BF00408370. [DOI] [PubMed] [Google Scholar]
- van der Zwaan J. W., Albracht S. P., Fontijn R. D., Slater E. C. Monovalent nickel in hydrogenase from Chromatium vinosum. Light sensitivity and evidence for direct interaction with hydrogen. FEBS Lett. 1985 Jan 7;179(2):271–277. doi: 10.1016/0014-5793(85)80533-0. [DOI] [PubMed] [Google Scholar]