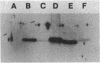
Abstract
The functionality of the Streptomyces lividans beta-galactosidase signal peptide to direct heterologous protein export was examined. The signal peptide plus eight amino acids of mature protein were sufficient to export not only a naturally exported protein, interleukin-1 beta, but also a naturally occurring cytoplasmic protein, Escherichia coli galactokinase. Interestingly, cells which expressed yet exported galactokinase were phenotypically Gal-. The potential use of the exported galactokinase system for the isolation and characterization of mutations within signal peptides and the export machinery of the host is discussed.
Full text
PDF
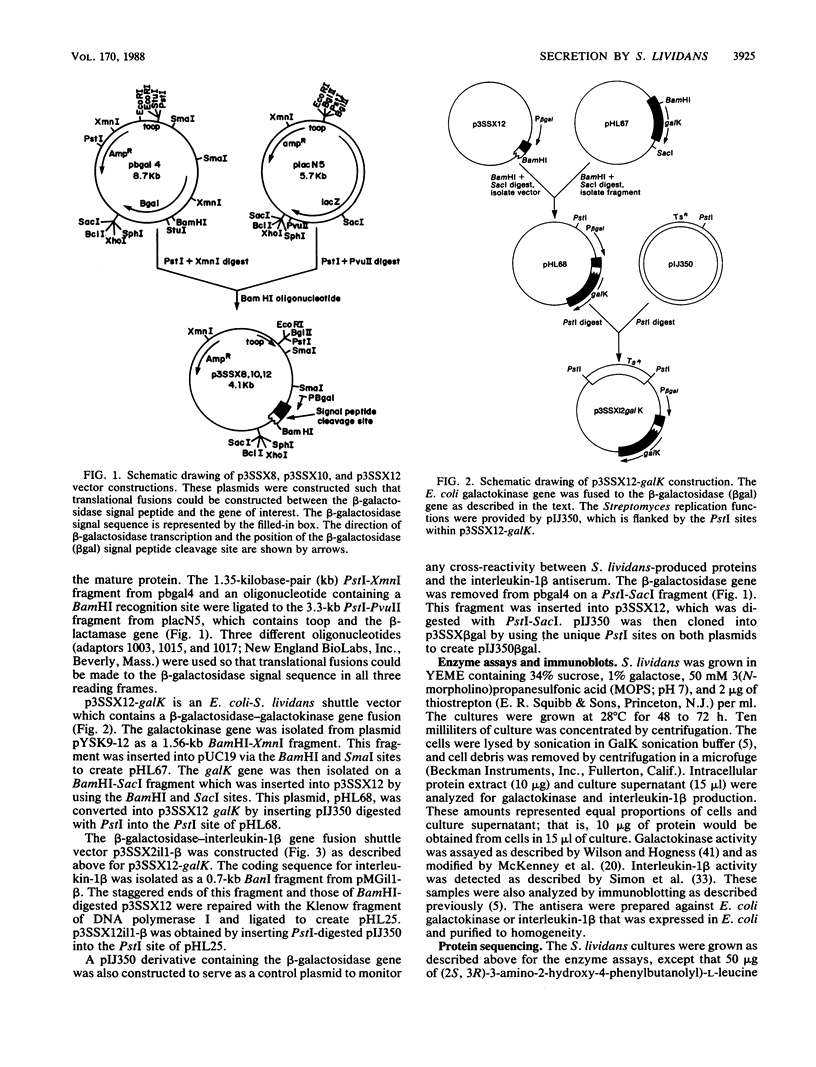
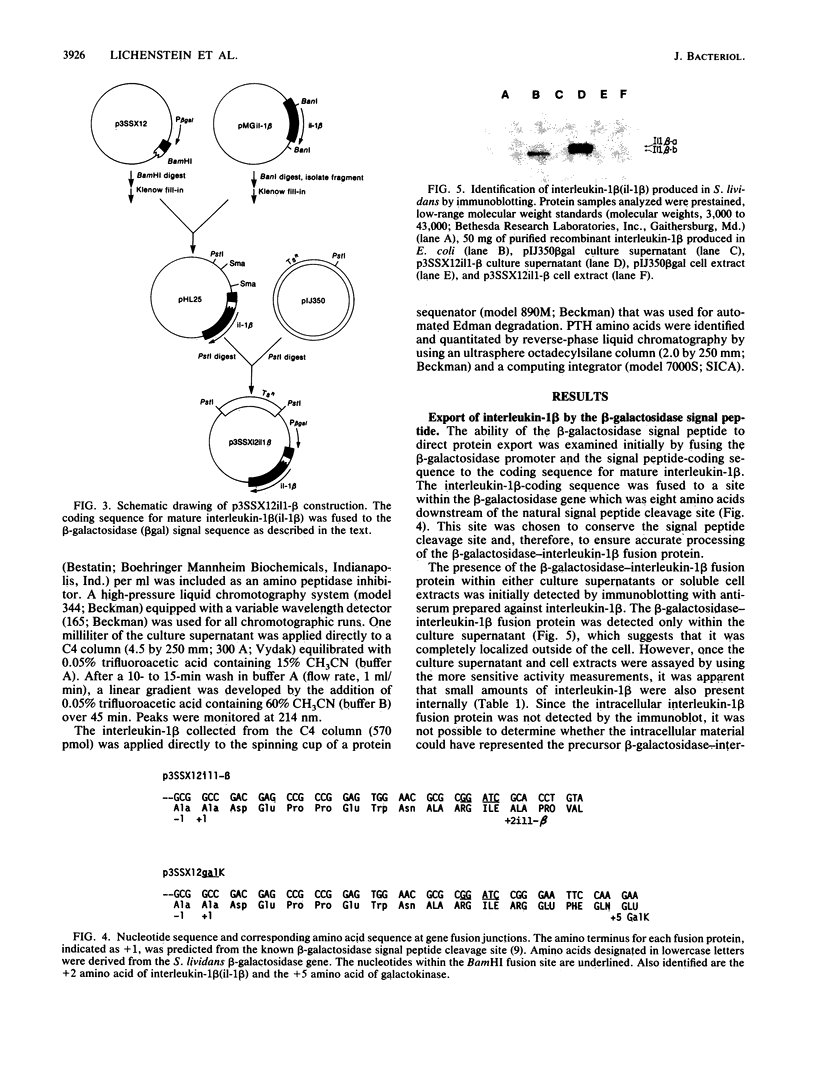



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S. A., Silhavy T. J. Information within the mature LamB protein necessary for localization to the outer membrane of E coli K12. Cell. 1983 Apr;32(4):1325–1335. doi: 10.1016/0092-8674(83)90313-6. [DOI] [PubMed] [Google Scholar]
- Brawner M. E., Auerbach J. I., Fornwald J. A., Rosenberg M., Taylor D. P. Characterization of Streptomyces promoter sequences using the Escherichia coli galactokinase gene. Gene. 1985;40(2-3):191–201. doi: 10.1016/0378-1119(85)90042-3. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Eckhardt T., Strickler J., Gorniak L., Burnett W. V., Fare L. R. Characterization of the promoter, signal sequence, and amino terminus of a secreted beta-galactosidase from "Streptomyces lividans". J Bacteriol. 1987 Sep;169(9):4249–4256. doi: 10.1128/jb.169.9.4249-4256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Silhavy T. J. Sequence information required for protein translocation from the cytoplasm. J Bacteriol. 1987 Dec;169(12):5339–5342. doi: 10.1128/jb.169.12.5339-5342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser T. H., Bruce B. J. Chicken ovalbumin is synthesized and secreted by Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5936–5940. doi: 10.1073/pnas.75.12.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G., Selzer G., Buell G., Shaw P., Escanez S., Hofer S., Voegeli P., Thompson C. J. Synthesis of bovine growth hormone by Streptomyces lividans. Gene. 1984 Dec;32(1-2):21–30. doi: 10.1016/0378-1119(84)90028-3. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Schwartz M., Silhavy T. J. Sequence information within the lamB genes in required for proper routing of the bacteriophage lambda receptor protein to the outer membrane of Escherichia coli K-12. J Mol Biol. 1982 Mar 25;156(1):93–112. doi: 10.1016/0022-2836(82)90461-2. [DOI] [PubMed] [Google Scholar]
- Hoshiko S., Makabe O., Nojiri C., Katsumata K., Satoh E., Nagaoka K. Molecular cloning and characterization of the Streptomyces hygroscopicus alpha-amylase gene. J Bacteriol. 1987 Mar;169(3):1029–1036. doi: 10.1128/jb.169.3.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T., Hopwood D. A., Wright H. M., Thompson C. J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185(2):223–228. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laluce C., Molinari R. Selection of a chemically defined medium for submerged cultivation of Streptomyces aureofaciens with high extracellular caseinolytic activity. Biotechnol Bioeng. 1977 Dec;19(12):1863–1884. doi: 10.1002/bit.260191210. [DOI] [PubMed] [Google Scholar]
- Lomovskaya N. D., Mkrtumian N. M., Gostimskaya N. L., Danilenko V. N. Characterization of temperate actinophage phi C31 isolated from Streptomyces coelicolor A3(2). J Virol. 1972 Feb;9(2):258–262. doi: 10.1128/jvi.9.2.258-262.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Meyers C. A., Johanson K. O., Miles L. M., McDevitt P. J., Simon P. L., Webb R. L., Chen M. J., Holskin B. P., Lillquist J. S., Young P. R. Purification and characterization of human recombinant interleukin-1 beta. J Biol Chem. 1987 Aug 15;262(23):11176–11181. [PubMed] [Google Scholar]
- Moreno F., Fowler A. V., Hall M., Silhavy T. J., Zabin I., Schwartz M. A signal sequence is not sufficient to lead beta-galactosidase out of the cytoplasm. Nature. 1980 Jul 24;286(5771):356–359. doi: 10.1038/286356a0. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Identification of a new gene (secA) and gene product involved in the secretion of envelope proteins in Escherichia coli. J Bacteriol. 1982 May;150(2):686–691. doi: 10.1128/jb.150.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva I., Lehtovaara P., Käriäinen L., Sibakov M., Cantell K., Schein C. H., Kashiwagi K., Weissmann C. Secretion of interferon by Bacillus subtilis. Gene. 1983 May-Jun;22(2-3):229–235. doi: 10.1016/0378-1119(83)90107-5. [DOI] [PubMed] [Google Scholar]
- Pulido D., Jiménez A. Optimization of gene expression in Streptomyces lividans by a transcription terminator. Nucleic Acids Res. 1987 May 26;15(10):4227–4240. doi: 10.1093/nar/15.10.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986 Sep 12;46(6):921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Trimble R. B., Wirth D. F., Hering C., Maley F., Maley G. F., Das R., Gibson B. W., Royal N., Biemann K. Primary structure of the Streptomyces enzyme endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1984 Jun 25;259(12):7577–7583. [PubMed] [Google Scholar]
- Simon P. L., Laydon J. T., Lee J. C. A modified assay for interleukin-1 (IL-1). J Immunol Methods. 1985 Nov 28;84(1-2):85–94. doi: 10.1016/0022-1759(85)90417-x. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Kaufman J., Gilbert W. Bacteria mature preproinsulin to proinsulin. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3988–3992. doi: 10.1073/pnas.77.7.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Stahl S., Gilbert W. Eukaryotic signal sequence transports insulin antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3369–3373. doi: 10.1073/pnas.77.6.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Cabello F., Cohen S. N. Cloning and characterization of EcoRI and HindIII restriction endonuclease-generated fragments of antibiotic resistance plasmids R6-5 and R6. Mol Gen Genet. 1978 Jun 14;162(2):121–137. doi: 10.1007/BF00267869. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Williams S. T., Goodfellow M., Alderson G., Wellington E. M., Sneath P. H., Sackin M. J. Numerical classification of Streptomyces and related genera. J Gen Microbiol. 1983 Jun;129(6):1743–1813. doi: 10.1099/00221287-129-6-1743. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]