Abstract
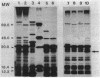
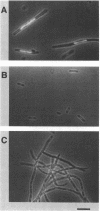
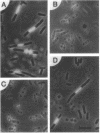
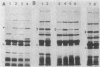
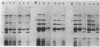
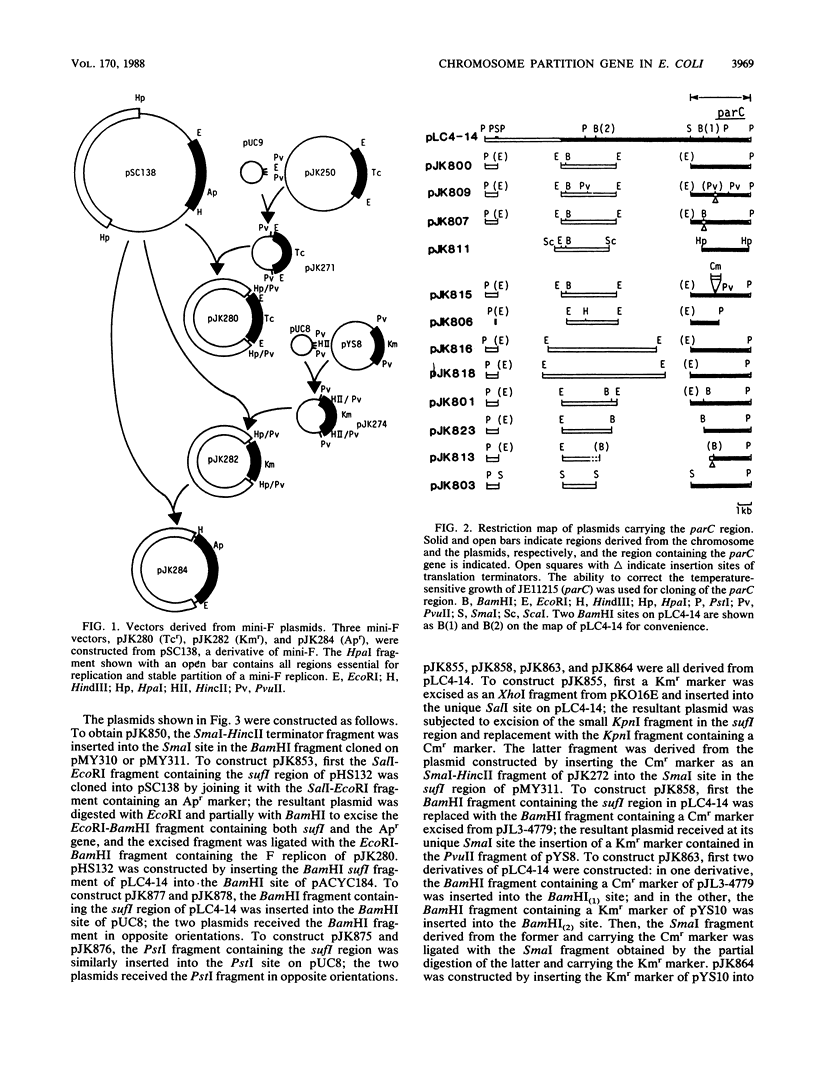
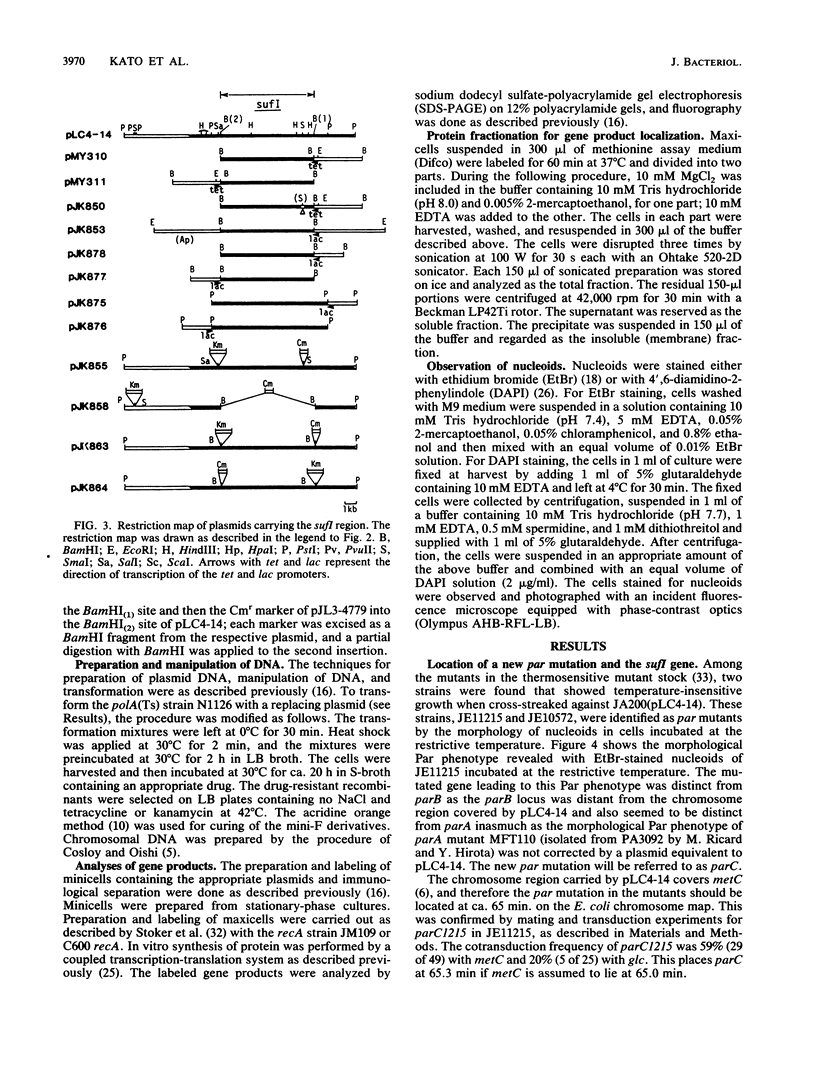
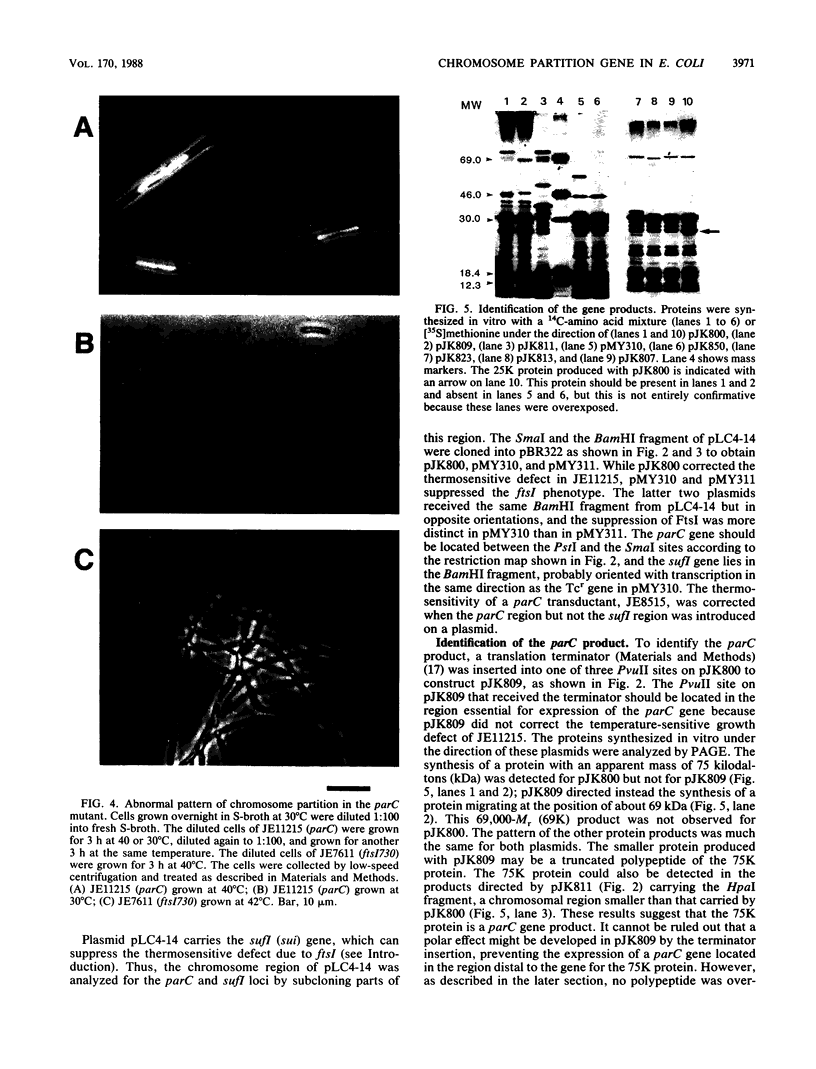
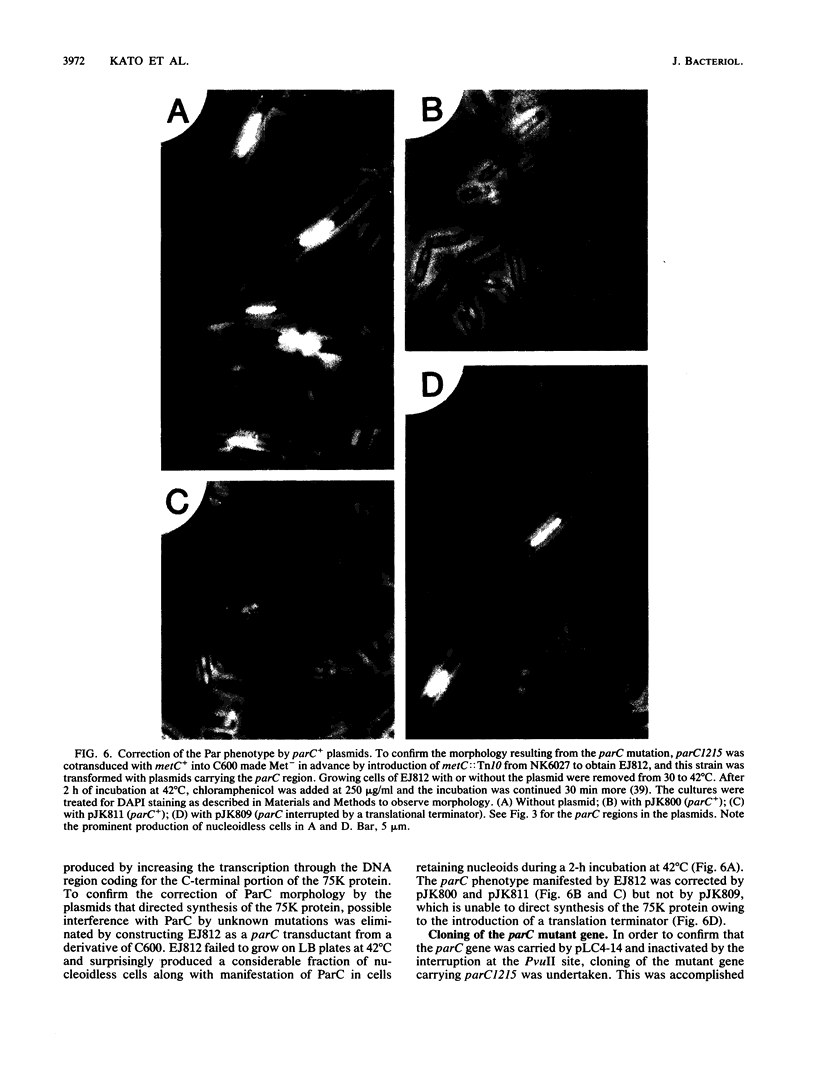
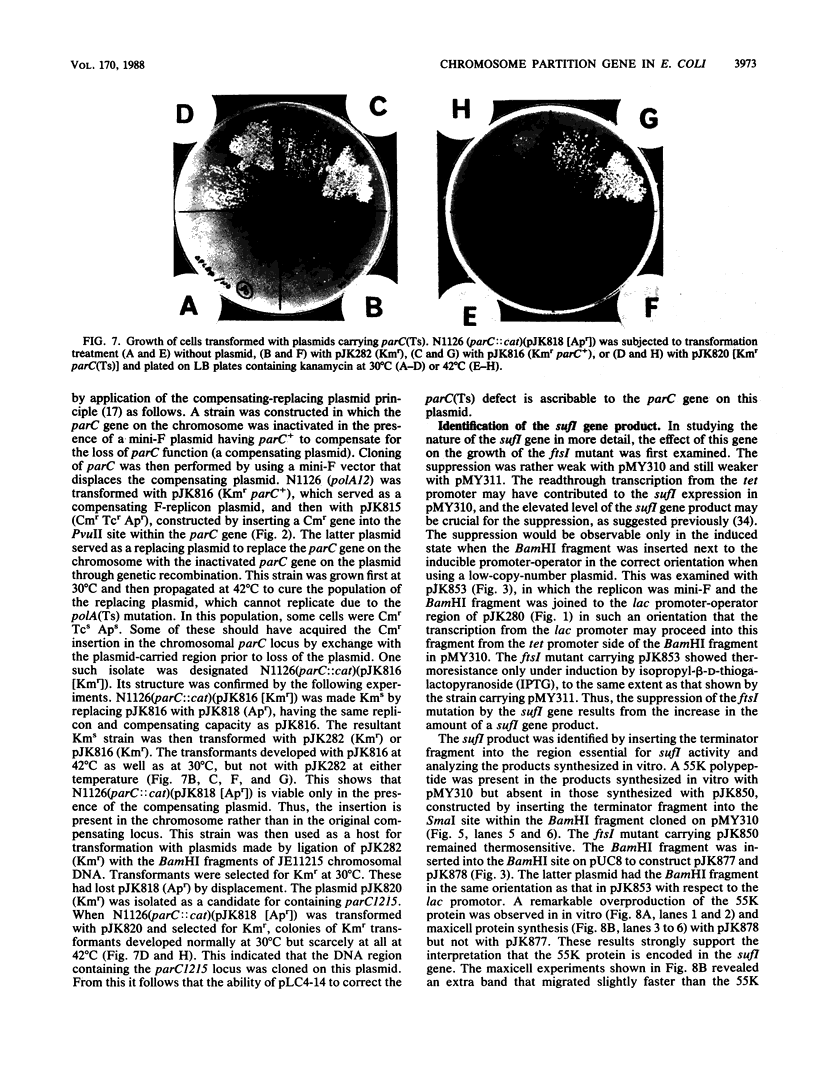
A new mutation, parC, causing abnormal chromosome segregation was identified in two thermosensitive mutants of Escherichia coli. The thermosensitive growth of the mutants was corrected by pLC4-14 in the Clarke-Carbon collection. This plasmid carries a putative gene which can suppress the cell division defect due to ftsI (pbpB) and has hence been termed sufI (sui). The nearness of parC to metC was confirmed, and cotransduction frequency of parC was 59% with metC and 20% with glc. The parC-sufI region was analyzed by subcloning the chromosome region of pLC4-14. The parC and the sufI gene products were electrophoretically identified as proteins of 75 and 55 kilodaltons (kDa), respectively. The allelism of parC+ on pLC4-14 to parC1215 was confirmed by cloning parC1215. The sufI gene appeared to be dispensable for cell viability, and overproduction of its product caused suppression of ftsI. An essential gene coding for a 25-kDa protein was found between the parC and the sufI gene. These three genes were transcribed in the same direction and may be organized into an operon, with parC to the proximal side and with internal promoters at least for the distal genes. The localization of the gene products was examined in maxicells. The sufI protein was synthesized as a precursor which could be chased into a mature form. The major part of the mature form was found in the soluble fraction. The 25-kDa protein was found almost exclusively in the membrane fraction. The parC protein was associated with the membrane fraction in the presence of Mg2+ but found in the soluble fraction when Mg2+ was sequestered with EDTA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta G. A., Park J. T. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J Bacteriol. 1981 Jan;145(1):333–340. doi: 10.1128/jb.145.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Biochemical construction and selection of hybrid plasmids containing specific segments of the Escherichia coli genome. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4361–4365. doi: 10.1073/pnas.72.11.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., Oishi M. The nature of the transformation process in Escherichia coli K12. Mol Gen Genet. 1973 Jul 31;124(1):1–10. doi: 10.1007/BF00267159. [DOI] [PubMed] [Google Scholar]
- Dwivedi C. M., Ragin R. C., Uren J. R. Cloning, purification, and characterization of beta-cystathionase from Escherichia coli. Biochemistry. 1982 Jun 22;21(13):3064–3069. doi: 10.1021/bi00256a005. [DOI] [PubMed] [Google Scholar]
- Harayama S., Hazelbauer G. L. A gene coding for a periplasmic protein is located near the locus for termination of chromosome replication in Escherichia coli. J Bacteriol. 1982 Sep;151(3):1391–1396. doi: 10.1128/jb.151.3.1391-1396.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y., Murotsu T., Matsubara K. Mini-F protein that binds to a unique region for partition of mini-F plasmid DNA. J Bacteriol. 1985 Jul;163(1):349–354. doi: 10.1128/jb.163.1.349-354.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. G., Kusano T., Yamaki H., Balakrishnan R., King M., Murchie J., Schaechter M. Binding of the origin of replication of Escherichia coli to the outer membrane. Cell. 1982 Oct;30(3):915–923. doi: 10.1016/0092-8674(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Further characterization of sfiA and sfiB mutations in Escherichia coli. J Bacteriol. 1980 Oct;144(1):185–191. doi: 10.1128/jb.144.1.185-191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., Gottesman S. Cell-division control in Escherichia coli: specific induction of the SOS function SfiA protein is sufficient to block septation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Suzuki H., Hirota Y. Dispensability of either penicillin-binding protein-1a or -1b involved in the essential process for cell elongation in Escherichia coli. Mol Gen Genet. 1985;200(2):272–277. doi: 10.1007/BF00425435. [DOI] [PubMed] [Google Scholar]
- Kato J., Suzuki H., Hirota Y. Overlapping of the coding regions for alpha and gamma components of penicillin-binding protein 1 b in Escherichia coli. Mol Gen Genet. 1984;196(3):449–457. doi: 10.1007/BF00436192. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T., Nishibayashi S., Kawano S., Suzuki T. Visualization of DNA in various phages (T4, chi, T7, phi 29) by ethidium bromide epi-fluorescent microscopy. Experientia. 1981;37(9):969–971. doi: 10.1007/BF01971784. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J Bacteriol. 1983 Jun;154(3):1339–1346. doi: 10.1128/jb.154.3.1339-1346.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacock P. A., Cohen S. N. Partitioning of bacterial plasmids during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell. 1980 Jun;20(2):529–542. doi: 10.1016/0092-8674(80)90639-x. [DOI] [PubMed] [Google Scholar]
- Miki T., Easton A. M., Rownd R. H. Cloning of replication, incompatibility, and stability functions of R plasmid NR1. J Bacteriol. 1980 Jan;141(1):87–99. doi: 10.1128/jb.141.1.87-99.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo A., Ohshima A., Ogura T., Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986 Nov 5;192(1):1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- Nagai K., Hendrickson W., Balakrishnan R., Yamaki H., Boyd D., Schaechter M. Isolation of a replication origin complex from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):262–266. doi: 10.1073/pnas.77.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takeda Y., Nishimura A., Suzuki H., Inouye M., Hirota Y. Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid. 1977 Nov;1(1):67–77. doi: 10.1016/0147-619x(77)90009-9. [DOI] [PubMed] [Google Scholar]
- Nordström K., Molin S., Aagaard-Hansen H. Partitioning of plasmid R1 in Escherichia coli. I. Kinetics of loss of plasmid derivatives deleted of the par region. Plasmid. 1980 Sep;4(2):215–227. doi: 10.1016/0147-619x(80)90011-6. [DOI] [PubMed] [Google Scholar]
- Norris V., Alliotte T., Jaffé A., D'Ari R. DNA replication termination in Escherichia coli parB (a dnaG allele), parA, and gyrB mutants affected in DNA distribution. J Bacteriol. 1986 Nov;168(2):494–504. doi: 10.1128/jb.168.2.494-504.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Nishimura A., Nishimura Y., Yamada M., Yasuda S., Suzuki H., Hirota Y. Synthetic ColE1 Plasmids carrying genes for penicillin-binding proteins in Escherichia coli. Plasmid. 1981 Jul;6(1):86–98. doi: 10.1016/0147-619x(81)90056-1. [DOI] [PubMed] [Google Scholar]
- Tanimoto K., Iino T. An essential gene for replication of the mini-F plasmid from origin I. Mol Gen Genet. 1984;196(1):59–63. doi: 10.1007/BF00334092. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zusman D. R., Carbonell A., Haga J. Y. Nucleoid condensation and cell division in Escherichia coli MX74T2 ts52 after inhibition of protein synthesis. J Bacteriol. 1973 Sep;115(3):1167–1178. doi: 10.1128/jb.115.3.1167-1178.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]