Abstract
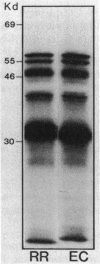
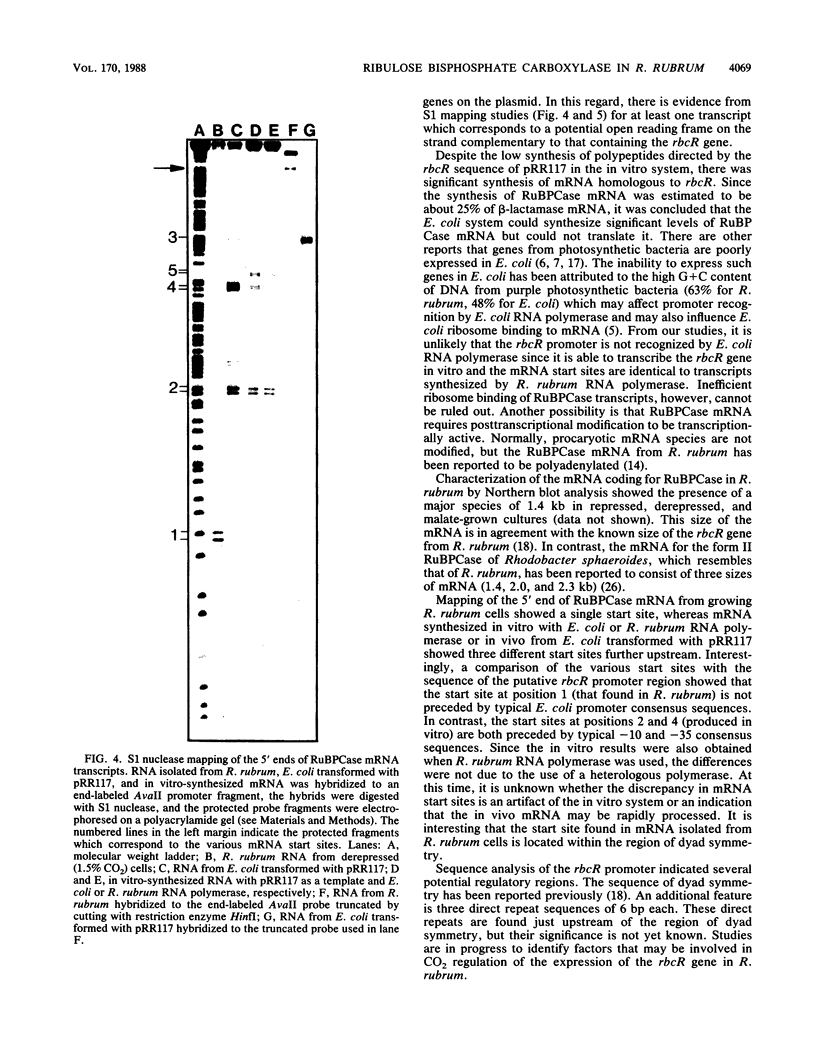
The synthesis of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBPCase) in Rhodospirillum rubrum was regulated by the CO2 concentration in the culture medium. The specific activity of RuBPCase in cells grown photolithotrophically in low concentrations of CO2 (1.5%) was five to ten times higher than that in cultures grown at high concentrations of CO2 (10%). Increased enzyme activity was reflected by an increase in both RuBPCase mRNA and RuBPCase protein. RuBPCase expression was also studied in vitro with a plasmid-borne genomic clone (pRR117) as the template in a partially defined Escherichia coli system containing either E. coli or R. rubrum RNA polymerase. With both enzymes there was excellent synthesis of RuBPCase mRNA, but no significant synthesis of RuBPCase was detected. The promoter region of the RuBPCase gene was sequenced, and mRNA start sites were mapped. A single major in vivo transcriptional start site was detected in RuBPCase mRNA extracted from R. rubrum. However, transcripts synthesized from pRR117 in vitro or from E. coli transformed with pRR117 started at upstream sites that were different from the in vivo transcription site. Two major features of the RuBPCase promoter region are three 6-base-pair direct repeats and a 31-base-pair region of dyad symmetry.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Organization of phosphoribulokinase and ribulose bisphosphate carboxylase/oxygenase genes in Rhodopseudomonas (Rhodobacter) sphaeroides. J Bacteriol. 1987 Aug;169(8):3685–3690. doi: 10.1128/jb.169.8.3685-3690.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. L., Kaplan S. Cloning of the gene for phosphoribulokinase activity from Rhodobacter sphaeroides and its expression in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3669–3678. doi: 10.1128/jb.169.8.3669-3678.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine G., Nash B., Weissbach H., Brot N. Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: Evidence for translational control. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5690–5694. doi: 10.1073/pnas.82.17.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1986 Feb;165(2):620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. F., Redfield B., Treadwell B. V., Eskin B., Spears C., Weissbach H. DNA-directed in vitro synthesis of beta-galactosidase. Studies with purified factors. J Biol Chem. 1977 Oct 10;252(19):6889–6894. [PubMed] [Google Scholar]
- Kung H., Spears C., Weissbach H. Purification and properties of a soluble factor required for the deoxyribonucleic acid-directed in vitro synthesis of beta-galactosidase. J Biol Chem. 1975 Feb 25;250(4):1556–1562. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T. Use of recombinant DNA technology to program eukaryotic cells to synthesize rat proinsulin: a rapid expression assay for cloned genes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5798–5802. doi: 10.1073/pnas.79.19.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar P. K., McFadden B. A. Polyadenylated mRNA from the photosynthetic procaryote Rhodospirillum rubrum. J Bacteriol. 1984 Mar;157(3):795–801. doi: 10.1128/jb.157.3.795-801.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Muller E. D., Chory J., Kaplan S. Cloning and characterization of the gene product of the form II ribulose-1,5-bisphosphate carboxylase gene of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jan;161(1):469–472. doi: 10.1128/jb.161.1.469-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarles L. S., Tabita F. R. Derepression of the synthesis of D-ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J Bacteriol. 1983 Jan;153(1):458–464. doi: 10.1128/jb.153.1.458-464.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]
- Zarucki-Schulz T., Jerez C., Goldberg G., Kung H. F., Huang K. H., Brot N., Weissbach H. DNA-directed in vitro synthesis of proteins involved in bacterial transcription and translation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6115–6119. doi: 10.1073/pnas.76.12.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kung S. D., Bogorad L. Phytochrome control of levels of mRNA complementary to plastid and nuclear genes of maize. Plant Physiol. 1985 Oct;79(2):371–376. doi: 10.1104/pp.79.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]