Abstract
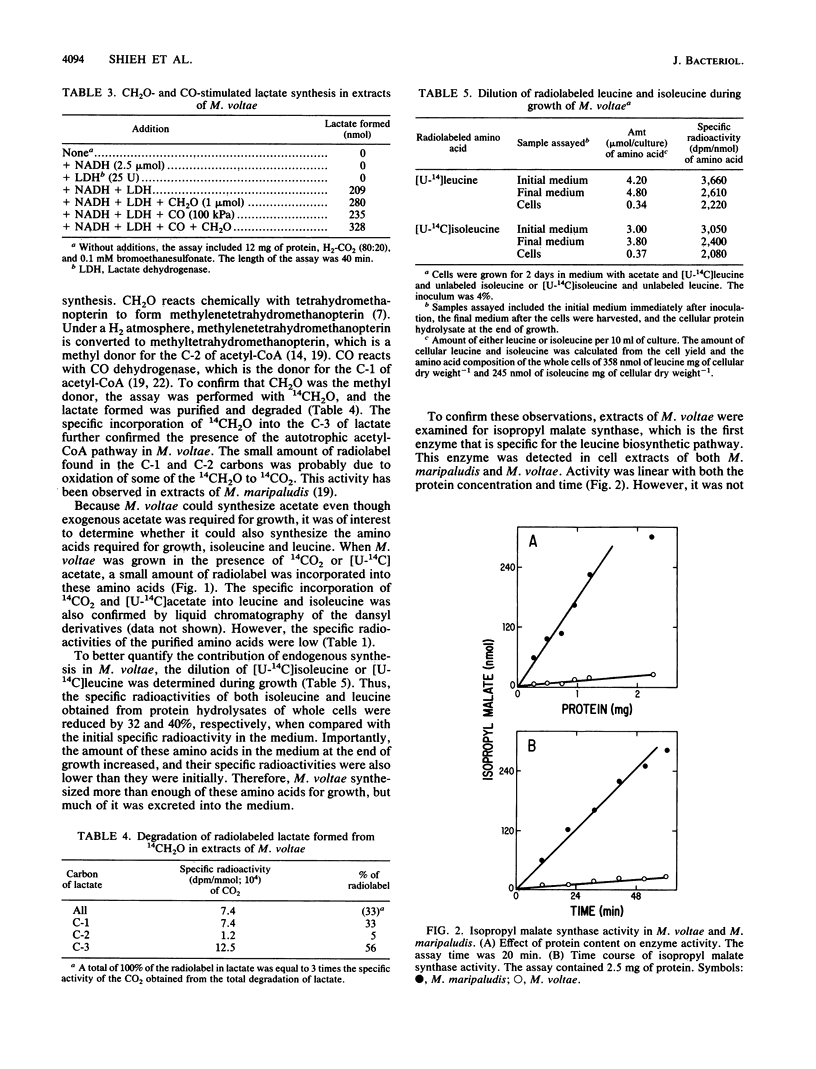
Methanococcus voltae is a methanogenic bacterium which requires leucine, isoleucine, and acetate for growth. However, it also can synthesize these amino acids, and it is capable of low levels of autotrophic acetyl coenzyme A (acetyl-CoA) biosynthesis. When cells were grown in the presence of 14CO2, as well as in the presence of compounds required for growth, the alanine found in the cellular protein was radiolabeled. The percentages of radiolabel in the C-1, C-2, and C-3 positions of alanine were 64, 24, and 16%, respectively. The incorporation of radiolabel into the C-2 and C-3 positions of alanine demonstrated the autotrophic acetyl-CoA biosynthetic pathway in this bacterium. Additional evidence was obtained in cell extracts in which autotrophically synthesized acetyl-CoA was trapped into lactate. In these extracts, both CO and CH2O stimulated acetyl-CoA synthesis. 14CH2O was specifically incorporated into the C-3 of lactate. Cell extracts of M. voltae also contained low levels of CO dehydrogenase, 13 nmol min-1 mg of protein-1. These results further confirmed the presence of the autotrophic acetyl-CoA biosynthetic pathway in M. voltae. Likewise, 14CO2 and [U-14C]acetate were also incorporated into leucine and isoleucine during growth. During growth with [U-14C]leucine or [U-14C]isoleucine, the specific radioactivity of these amino acids in the culture medium declined, and the specific radioactivities of these amino acids recovered from the cellular protein were 32 to 40% lower than the initial specific radioactivities in the medium.Cell extracts of M. voltae also contained levels of isopropyl malate synthase, an enzyme that is specific to the leucine biosynthetic pathway, of 0.8 nmol min-1 mg of protein-1. Thus, M. voltae is capable of autotrophic CO2 fixation and leucine and isoleucine biosynthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Stedman J. D. Efficient fluorography of 3H and 14C on thin layers. Anal Biochem. 1978 Aug 15;89(1):247–256. doi: 10.1016/0003-2697(78)90747-9. [DOI] [PubMed] [Google Scholar]
- Calvo J. M., Bartholomew J. C., Stieglitz B. I. Fluorometric assay of enzymatic reactions involving acetyl Coenzyme A in aldol condensations. Anal Biochem. 1969 Apr 4;28(1):164–181. doi: 10.1016/0003-2697(69)90168-7. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Jarrell K. F., Sprott G. D. Amino acid biosynthesis and sodium-dependent transport in Methanococcus voltae, as revealed by 13C NMR. Eur J Biochem. 1985 Jun 3;149(2):437–444. doi: 10.1111/j.1432-1033.1985.tb08944.x. [DOI] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Wolfe R. S. Tetrahydromethanopterin-dependent methanogenesis from non-physiological C1 donors in Methanobacterium thermoautotrophicum. J Bacteriol. 1985 Feb;161(2):696–701. doi: 10.1128/jb.161.2.696-701.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E., Thauer R. K. Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Apr 27;117(1):61–66. doi: 10.1007/BF00689352. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Länge S., Fuchs G. Autotrophic synthesis of activated acetic acid from CO2 in Methanobacterium thermoautotrophicum. Synthesis from tetrahydromethanopterin-bound C1 units and carbon monoxide. Eur J Biochem. 1987 Feb 16;163(1):147–154. doi: 10.1111/j.1432-1033.1987.tb10748.x. [DOI] [PubMed] [Google Scholar]
- Shieh J. S., Whitman W. B. Pathway of acetate assimilation in autotrophic and heterotrophic methanococci. J Bacteriol. 1987 Nov;169(11):5327–5329. doi: 10.1128/jb.169.11.5327-5329.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J., Whitman W. B. Autotrophic acetyl coenzyme A biosynthesis in Methanococcus maripaludis. J Bacteriol. 1988 Jul;170(7):3072–3079. doi: 10.1128/jb.170.7.3072-3079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Usher J. R. The electrochemical proton gradient and phenylalanine transport in Escherichia coli irradiated with near-ultraviolet light. Can J Microbiol. 1977 Dec;23(12):1683–1688. doi: 10.1139/m77-242. [DOI] [PubMed] [Google Scholar]
- Stupperich E., Hammel K. E., Fuchs G., Thauer R. K. Carbon monoxide fixation into the carboxyl group of acetyl coenzyme A during autotrophic growth of Methanobacterium. FEBS Lett. 1983 Feb 7;152(1):21–23. doi: 10.1016/0014-5793(83)80473-6. [DOI] [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Sohn S., Kuk S., Xing R. Role of Amino Acids and Vitamins in Nutrition of Mesophilic Methanococcus spp. Appl Environ Microbiol. 1987 Oct;53(10):2373–2378. doi: 10.1128/aem.53.10.2373-2378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F. Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol. 1986 May;51(5):1056–1062. doi: 10.1128/aem.51.5.1056-1062.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel J., Schlegel H. G. Alpha-Isopropylmalate synthase from Alcaligenes eutrophus H 16 I. Purification and general properties. Arch Microbiol. 1977 Apr 1;112(3):239–246. doi: 10.1007/BF00413087. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Schlegel H. G. alpha-Isopropylmalate synthase from Alcaligenes eutrophus H 16. II. Substrate specificity and kinetics. Arch Microbiol. 1977 Apr 1;112(3):247–254. doi: 10.1007/BF00413088. [DOI] [PubMed] [Google Scholar]
- Xing R. Y., Whitman W. B. Sulfometuron methyl-sensitive and -resistant acetolactate synthases of the archaebacteria Methanococcus spp. J Bacteriol. 1987 Oct;169(10):4486–4492. doi: 10.1128/jb.169.10.4486-4492.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]