Abstract
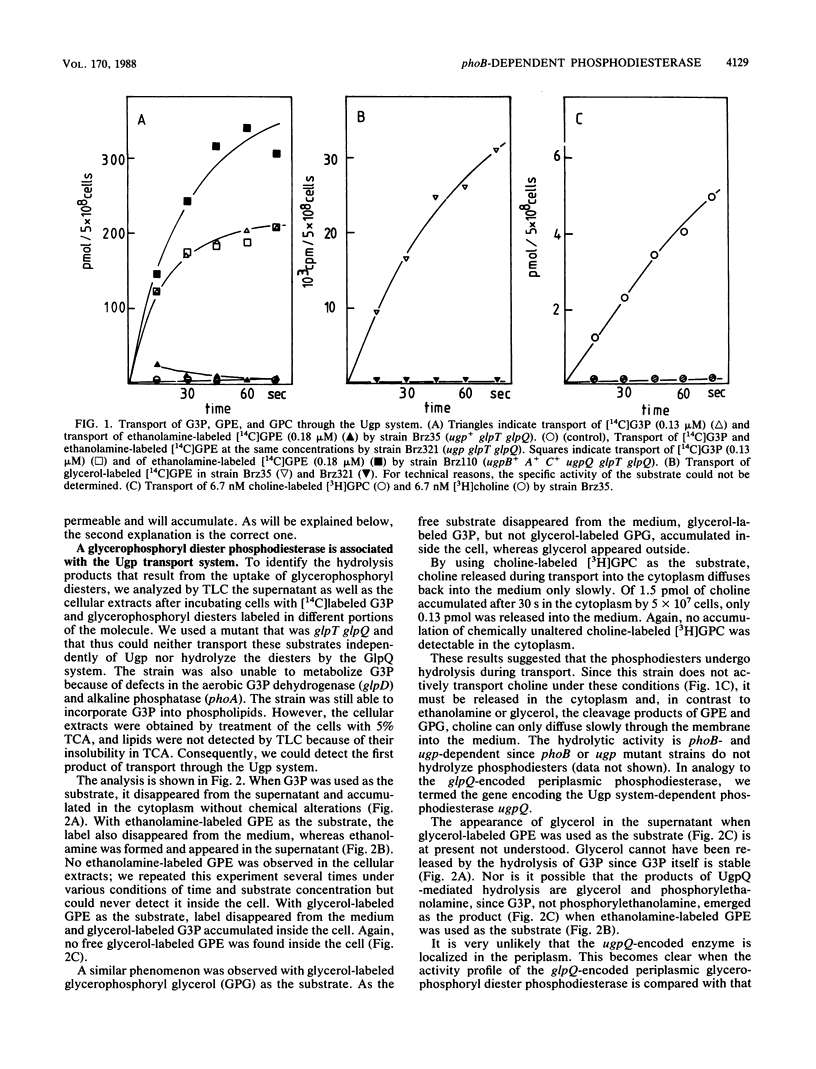
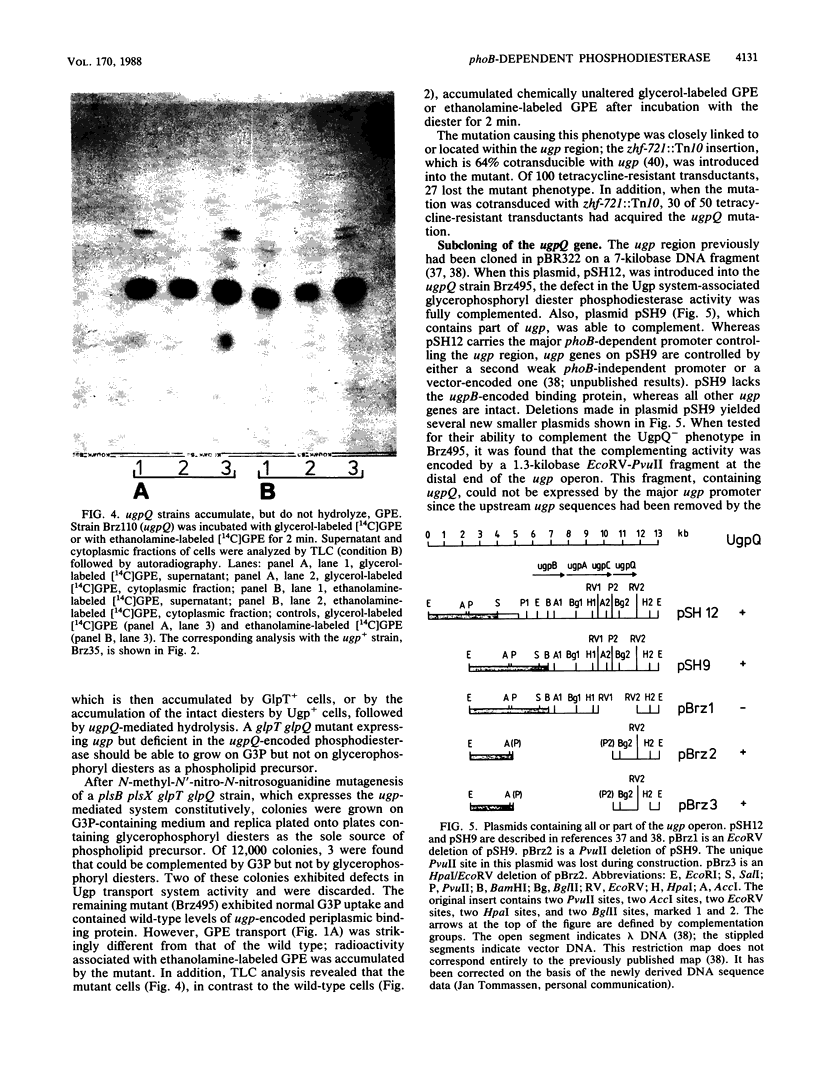
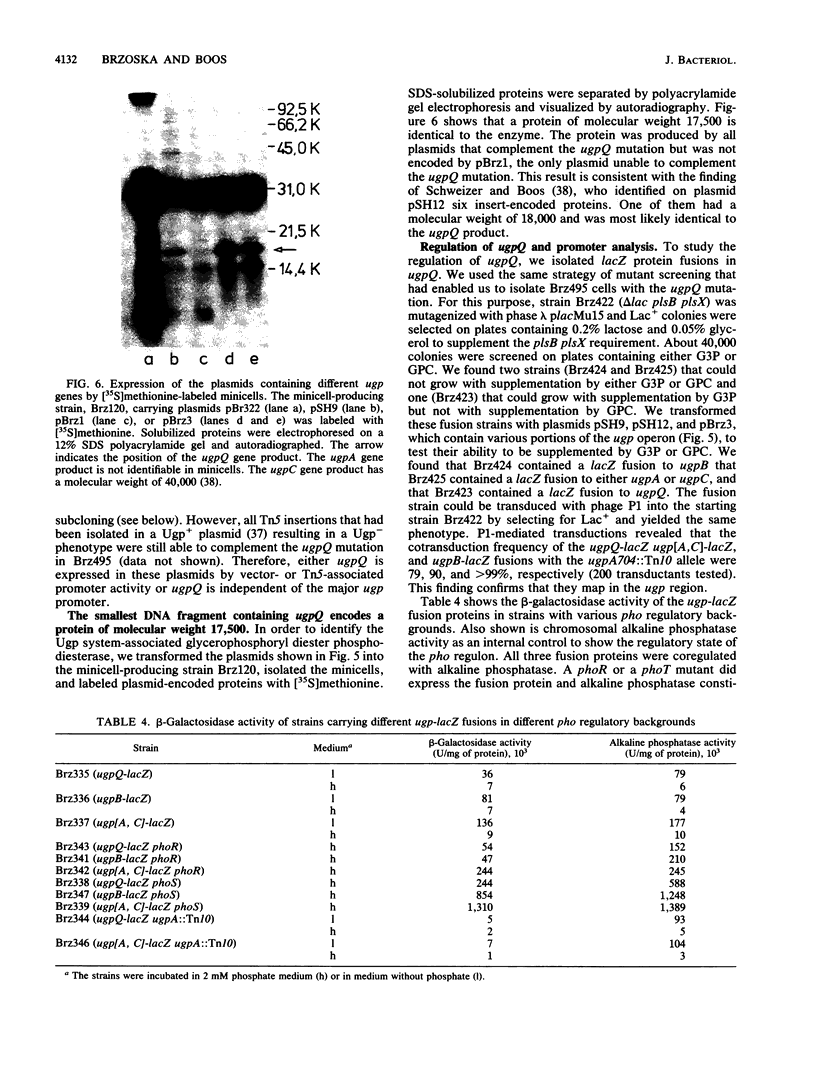
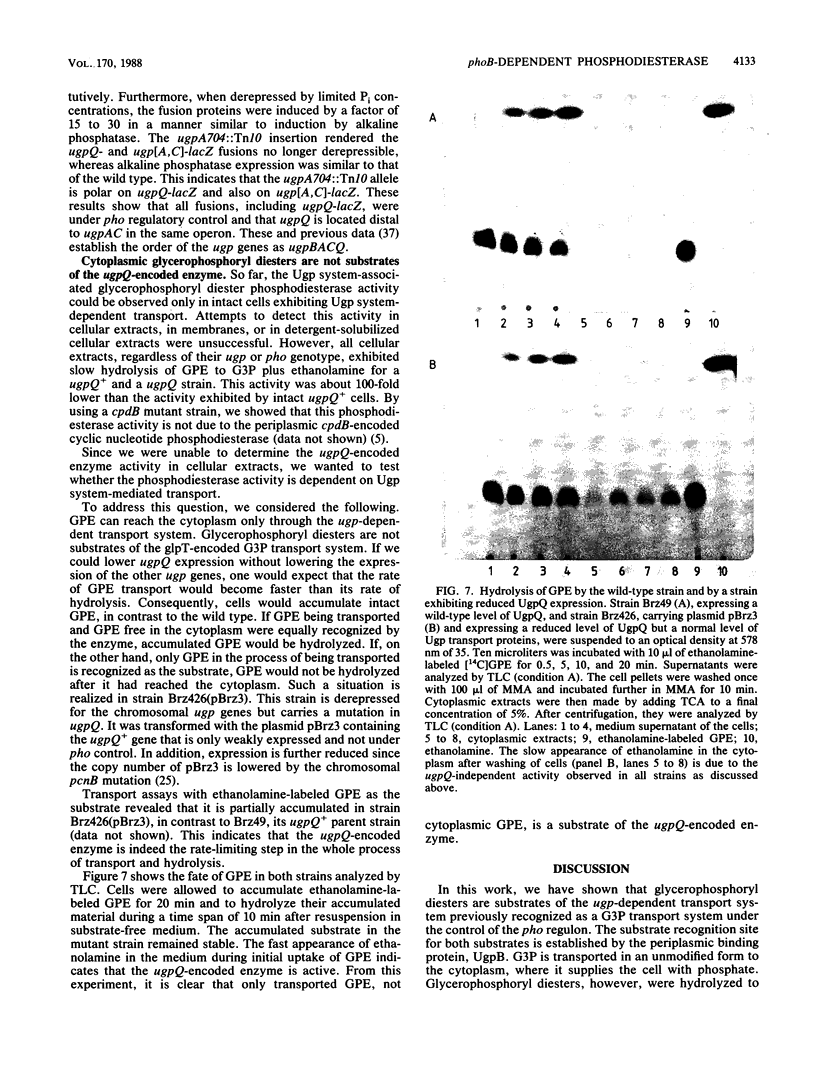
The ugp-encoded transport system of Escherichia coli accumulates sn-glycerol-3-phosphate with high affinity; it is binding protein mediated and part of the pho regulon. Here, we report that glycerophosphoryl diesters (deacylated phospholipids) are also high-affinity substrates for the ugp-encoded system. The diesters are not taken up in an unaltered form but are hydrolyzed during transport to sn-glycerol-3-phosphate plus the corresponding alcohols. The enzyme responsible for this reaction is not essential for the translocation of sn-glycerol-3-phosphate or for the glycerophosphoryl diesters but can only hydrolyze diesters that are in the process of being transported. Diesters in the periplasm or in the cytoplasm were not recognized, and no enzymatic activity could be detected in cellular extracts. The enzyme is encoded by the last gene in the ugp operon, termed ugpQ. The product of the ugpQ gene, expressed in minicells, has an apparent molecular weight of 17,500. We present evidence that only one major phoB-dependent promoter controls all ugp genes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Larson T. J., Maloney P. C. Reconstitution of sugar phosphate transport systems of Escherichia coli. J Biol Chem. 1986 Jul 15;261(20):9083–9086. [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Argast M., Boos W. Purification and properties of the sn-glycerol 3-phosphate-binding protein of Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):10931–10935. [PubMed] [Google Scholar]
- Argast M., Ludtke D., Silhavy T. J., Boos W. A second transport system for sn-glycerol-3-phosphate in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1070–1083. doi: 10.1128/jb.136.3.1070-1083.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham I. R., Garrett S. Isolation of Escherichia coli mutants (cpdB) deficient in periplasmic 2':3'-cyclic phosphodiesterase and genetic mapping of the cpdB locus. J Gen Microbiol. 1980 Jul;119(1):31–34. doi: 10.1099/00221287-119-1-31. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Cronan J. E., Jr Mutants of Escherichia coli defective in membrane phospholipid synthesis. Phenotypic suppression of sn-glycerol-3-phosphate acyltransferase Km mutants by loss of feedback inhibition of the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J Biol Chem. 1975 Sep 25;250(18):7153–7158. [PubMed] [Google Scholar]
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bremer E., Silhavy T. J., Weisemann J. M., Weinstock G. M. Lambda placMu: a transposable derivative of bacteriophage lambda for creating lacZ protein fusions in a single step. J Bacteriol. 1984 Jun;158(3):1084–1093. doi: 10.1128/jb.158.3.1084-1093.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz G. W., Jr The hexose phosphate transport system of Escherichia coli. Adv Enzymol Relat Areas Mol Biol. 1976;44:237–259. doi: 10.1002/9780470122891.ch7. [DOI] [PubMed] [Google Scholar]
- Elvin C. M., Hardy C. M., Rosenberg H. Pi exchange mediated by the GlpT-dependent sn-glycerol-3-phosphate transport system in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1054–1058. doi: 10.1128/jb.161.3.1054-1058.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Hengge R., Boos W. Defective secretion of maltose- and ribose-binding proteins caused by a truncated periplasmic protein in Escherichia coli. J Bacteriol. 1985 Jun;162(3):972–978. doi: 10.1128/jb.162.3.972-978.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope J. N., Bell A. W., Hermodson M. A., Groarke J. M. Ribokinase from Escherichia coli K12. Nucleotide sequence and overexpression of the rbsK gene and purification of ribokinase. J Biol Chem. 1986 Jun 15;261(17):7663–7668. [PubMed] [Google Scholar]
- Kadner R. J., Shattuck-Eidens D. M. Genetic control of the hexose phosphate transport system of Escherichia coli: mapping of deletion and insertion mutations in the uhp region. J Bacteriol. 1983 Sep;155(3):1052–1061. doi: 10.1128/jb.155.3.1052-1061.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Ehrmann M., Boos W. Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem. 1983 May 10;258(9):5428–5432. [PubMed] [Google Scholar]
- Larson T. J., Ludtke D. N., Bell R. M. sn-Glycerol-3-phosphate auxotrophy of plsB strains of Escherichia coli: evidence that a second mutation, plsX, is required. J Bacteriol. 1984 Nov;160(2):711–717. doi: 10.1128/jb.160.2.711-717.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. J., Schumacher G., Boos W. Identification of the glpT-encoded sn-glycerol-3-phosphate permease of Escherichia coli, an oligomeric integral membrane protein. J Bacteriol. 1982 Dec;152(3):1008–1021. doi: 10.1128/jb.152.3.1008-1021.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. J., Ye S. Z., Weissenborn D. L., Hoffmann H. J., Schweizer H. Purification and characterization of the repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K12. J Biol Chem. 1987 Nov 25;262(33):15869–15874. [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Lopilato J., Bortner S., Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986 Nov;205(2):285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- Ludtke D., Larson T. J., Beck C., Boos W. Only one gene is required for the glpT-dependent transport of sn-glycerol-3-phosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):540–547. doi: 10.1007/BF00337962. [DOI] [PubMed] [Google Scholar]
- Magazin M., Howe M., Allet B. Partial correlation of the genetic and physical maps of bacteriophage Mu. Virology. 1977 Apr;77(2):677–688. doi: 10.1016/0042-6822(77)90491-3. [DOI] [PubMed] [Google Scholar]
- Nakata A., Amemura M., Shinagawa H. Regulation of the phosphate regulon in Escherichia coli K-12: regulation of the negative regulatory gene phoU and identification of the gene product. J Bacteriol. 1984 Sep;159(3):979–985. doi: 10.1128/jb.159.3.979-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Molecular genetics of membrane phospholipid synthesis. Annu Rev Genet. 1986;20:253–295. doi: 10.1146/annurev.ge.20.120186.001345. [DOI] [PubMed] [Google Scholar]
- Rao N. N., Wang E., Yashphe J., Torriani A. Nucleotide pool in pho regulon mutants and alkaline phosphatase synthesis in Escherichia coli. J Bacteriol. 1986 Apr;166(1):205–211. doi: 10.1128/jb.166.1.205-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme G., Kepes A. Study of binding protein-ligand interaction by ammonium sulfate-assisted adsorption on cellulose esters filters. Biochim Biophys Acta. 1983 Jan 12;742(1):16–24. doi: 10.1016/0167-4838(83)90353-9. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Argast M., Boos W. Characteristics of a binding protein-dependent transport system for sn-glycerol-3-phosphate in Escherichia coli that is part of the pho regulon. J Bacteriol. 1982 Jun;150(3):1154–1163. doi: 10.1128/jb.150.3.1154-1163.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Characterization of the ugp region containing the genes for the phoB dependent sn-glycerol-3-phosphate transport system of Escherichia coli. Mol Gen Genet. 1984;197(1):161–168. doi: 10.1007/BF00327937. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Cloning of the ugp region containing the structural genes for the pho regulon-dependent sn-glycerol-3-phosphate transport system of Escherichia coli. Mol Gen Genet. 1983;192(1-2):177–186. doi: 10.1007/BF00327664. [DOI] [PubMed] [Google Scholar]
- Schweizer H., Boos W. Regulation of ugp, the sn-glycerol-3-phosphate transport system of Escherichia coli K-12 that is part of the pho regulon. J Bacteriol. 1985 Jul;163(1):392–394. doi: 10.1128/jb.163.1.392-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Grussenmeyer T., Boos W. Mapping of two ugp genes coding for the pho regulon-dependent sn-glycerol-3-phosphate transport system of Escherichia coli. J Bacteriol. 1982 Jun;150(3):1164–1171. doi: 10.1128/jb.150.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surin B. P., Rosenberg H., Cox G. B. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J Bacteriol. 1985 Jan;161(1):189–198. doi: 10.1128/jb.161.1.189-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston L. A., Kadner R. J. Identification of uhp polypeptides and evidence for their role in exogenous induction of the sugar phosphate transport system of Escherichia coli K-12. J Bacteriol. 1987 Aug;169(8):3546–3555. doi: 10.1128/jb.169.8.3546-3555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]