Abstract
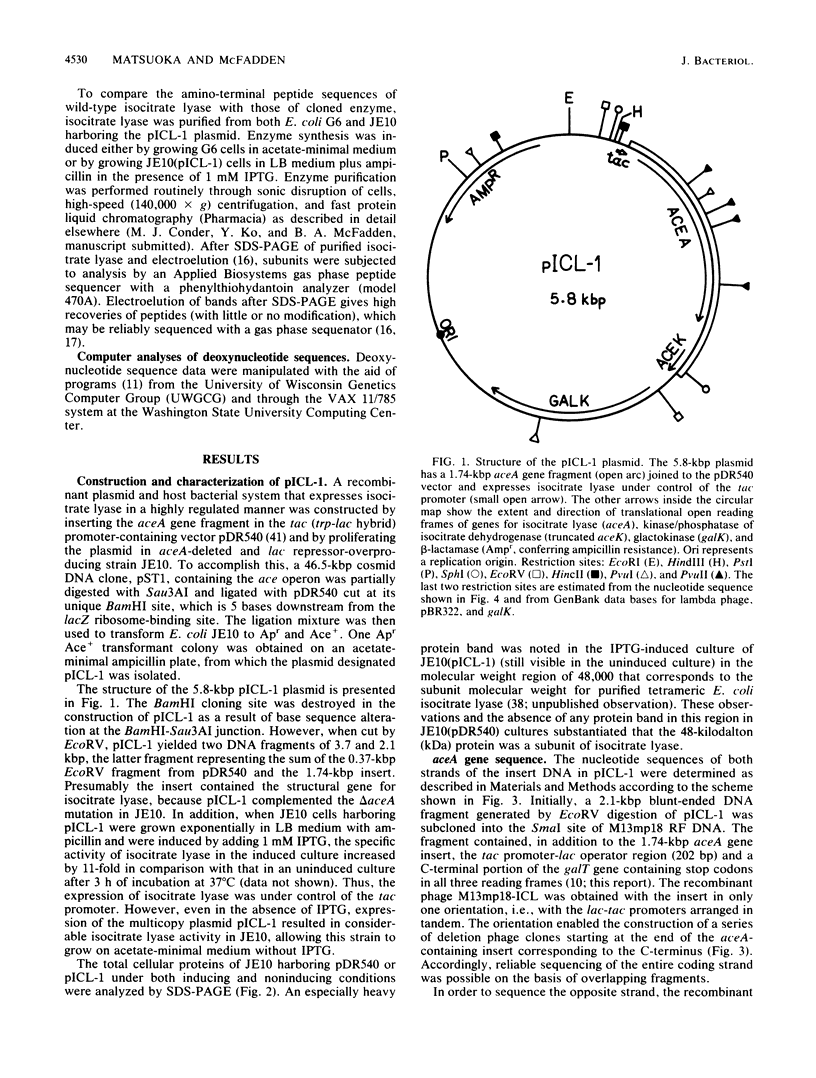
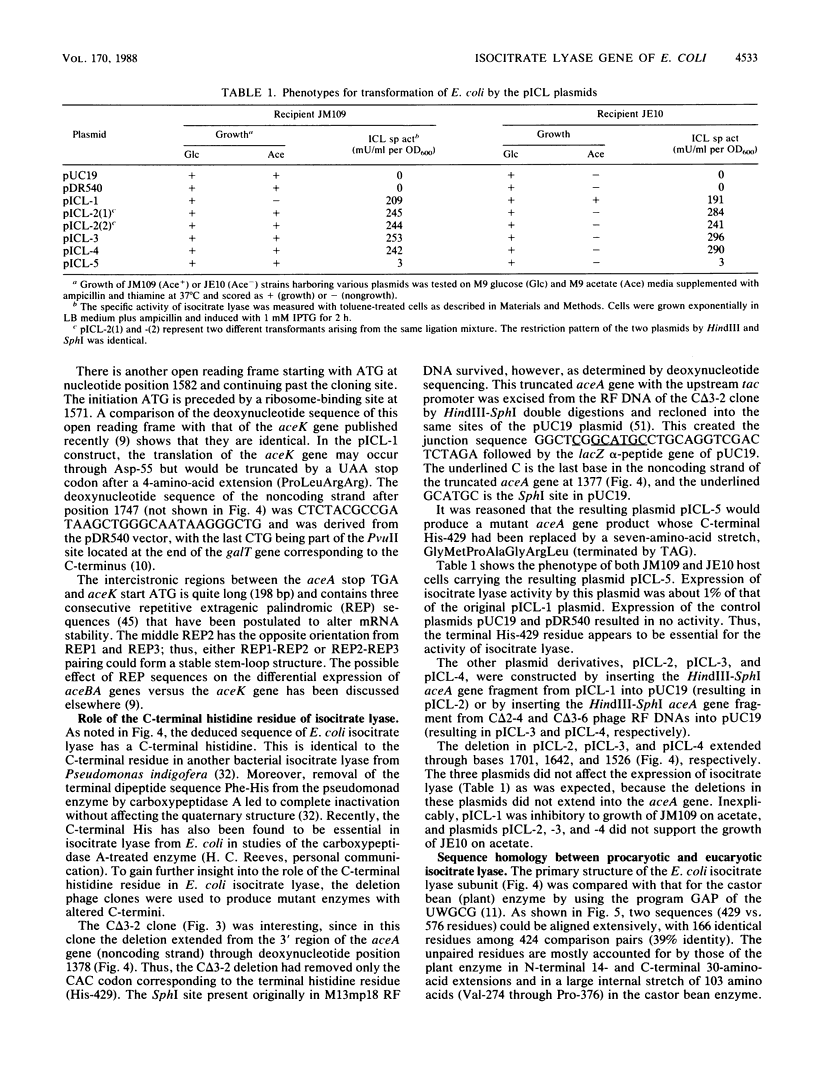
A structural gene for isocitrate lyase was isolated from a cosmid containing an ace locus of the Escherichia coli chromosome. Cloning and expression under control of the tac promoter in a multicopy plasmid showed that a 1.7-kilobase-pair DNA segment was sufficient for complementation of an aceA deletion mutation and overproduction of isocitrate lyase. DNA sequence analysis of the cloned gene and N-terminal protein sequencing of the cloned and wild-type enzymes revealed an entire aceA gene which encodes a 429-amino-acid residue polypeptide whose C-terminus is histidine. The deduced amino acid sequence for the 47.2-kilodalton subunit of E. coli isocitrate lyase could be aligned with that for the 64.8-kilodalton subunit of the castor bean enzyme with 39% identity except for limited N- and C-terminal regions and a 103-residue stretch that was unique for the plant enzyme and started approximately in the middle of that peptide.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley H. F., Kornberg H. L. Sequence homologies between proteins of bacterial phosphoenolpyruvate-dependent sugar phosphotransferase systems: identification of possible phosphate-carrying histidine residues. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4777–4780. doi: 10.1073/pnas.84.14.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice C. B., Kornberg H. L. Genetic control of isocitrate lyase activity in Escherichia coli. J Bacteriol. 1968 Dec;96(6):2185–2186. doi: 10.1128/jb.96.6.2185-2186.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck D., Spencer M. E., Guest J. R. Primary structure of the succinyl-CoA synthetase of Escherichia coli. Biochemistry. 1985 Oct 22;24(22):6245–6252. doi: 10.1021/bi00343a031. [DOI] [PubMed] [Google Scholar]
- Chung T., Klumpp D. J., LaPorte D. C. Glyoxylate bypass operon of Escherichia coli: cloning and determination of the functional map. J Bacteriol. 1988 Jan;170(1):386–392. doi: 10.1128/jb.170.1.386-392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortay J. C., Bleicher F., Rieul C., Reeves H. C., Cozzone A. J. Nucleotide sequence and expression of the aceK gene coding for isocitrate dehydrogenase kinase/phosphatase in Escherichia coli. J Bacteriol. 1988 Jan;170(1):89–97. doi: 10.1128/jb.170.1.89-97.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Jameel S., El-Gul T., McFadden B. A. Modification of the active site of isocitrate lyase from watermelon cotyledons. Arch Biochem Biophys. 1985 Jan;236(1):72–81. doi: 10.1016/0003-9861(85)90607-1. [DOI] [PubMed] [Google Scholar]
- Johanson R. A., Hill J. M., McFadden B. A. Isocitrate lyase from Neurospora crassa. II. Composition, quaternary structure, C-terminus, and active-site modification. Biochim Biophys Acta. 1974 Oct 17;364(2):341–352. doi: 10.1016/0005-2744(74)90019-9. [DOI] [PubMed] [Google Scholar]
- Khan F. R., Saleemuddin M., Siddiqi M., McFadden B. A. Purification and properties of isocitrate lyase from flax seedlings. Arch Biochem Biophys. 1977 Sep;183(1):13–23. doi: 10.1016/0003-9861(77)90413-1. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Ross D. G. Translocation and other recombination events involving the tetracycline-resistance element Tn10. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1233–1246. doi: 10.1101/sqb.1979.043.01.140. [DOI] [PubMed] [Google Scholar]
- Klumpp D. J., Plank D. W., Bowdin L. J., Stueland C. S., Chung T., LaPorte D. C. Nucleotide sequence of aceK, the gene encoding isocitrate dehydrogenase kinase/phosphatase. J Bacteriol. 1988 Jun;170(6):2763–2769. doi: 10.1128/jb.170.6.2763-2769.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature. 1982 Dec 2;300(5891):458–460. doi: 10.1038/300458a0. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Thorsness P. E., Koshland D. E., Jr Compensatory phosphorylation of isocitrate dehydrogenase. A mechanism for adaptation to the intracellular environment. J Biol Chem. 1985 Sep 5;260(19):10563–10568. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):173–180. doi: 10.1128/jb.149.1.173-180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Rao G. R., Cohen A. L., Roche T. E. Isocitrate lyase from Pseudomonas indigofera. V. Subunits and terminal residues and the relation to catalytic activity. Biochemistry. 1968 Oct;7(10):3574–3582. doi: 10.1021/bi00850a035. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Rose I. A., Williams J. O. Production of pyruvate and succinate by action of isocitrate lyase on -methylisocitrate. Arch Biochem Biophys. 1972 Jan;148(1):84–88. doi: 10.1016/0003-9861(72)90118-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nagahari K., Tanaka T., Hishinuma F., Kuroda M., Sakaguchi K. Control of tryptophan synthetase amplified by varying the numbers of composite plasmids in Escherichia coli cells. Gene. 1977 Mar;1(2):141–152. doi: 10.1016/0378-1119(77)90025-7. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Robertson E. F., Hoyt J. C., Reeves H. C. Evidence of histidine phosphorylation in isocitrate lyase from Escherichia coli. J Biol Chem. 1988 Feb 15;263(5):2477–2482. [PubMed] [Google Scholar]
- Roche T. E., McFadden B. A., Williams J. O. Modification of the active site of isocitrate lyase from Pseudomonas indigofera. Arch Biochem Biophys. 1971 Nov;147(1):192–200. doi: 10.1016/0003-9861(71)90327-4. [DOI] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Thorsness P. E., Koshland D. E., Jr Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem. 1987 Aug 5;262(22):10422–10425. [PubMed] [Google Scholar]
- Volokita M., Somerville C. R. The primary structure of spinach glycolate oxidase deduced from the DNA sequence of a cDNA clone. J Biol Chem. 1987 Nov 25;262(33):15825–15828. [PubMed] [Google Scholar]
- Wang T., Jurásek L., Bridger W. A. Succinyl coenzyme A synthetase of Escherichia coli. Sequence of a peptide containing the active-site phosphohistidine residue. Biochemistry. 1972 May 23;11(11):2067–2070. doi: 10.1021/bi00761a011. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Swinkels B., Michels P. A., Osinga K., Misset O., Van Beeumen J., Gibson W. C., Postma J. P., Borst P., Opperdoes F. R. Common elements on the surface of glycolytic enzymes from Trypanosoma brucei may serve as topogenic signals for import into glycosomes. EMBO J. 1987 Jan;6(1):215–221. doi: 10.1002/j.1460-2075.1987.tb04741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



