Abstract
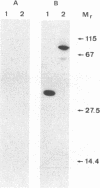
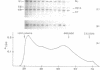
An endoxylanase encoded by the xynZ gene of Clostridium thermocellum was purified from Escherichia coli harbouring a fragment of the gene cloned in pUC8. The purified enzyme showed two active bands of Mr 41,000 and 39,000, the latter one presumably derived from the former through proteolysis. The enzyme was highly active on xylan and para-nitrophenyl-beta-D-xylobioside. The major end product of xylan hydrolysis was xylobiose. With an antiserum raised against the enzyme purified from E. coli, an immunoreactive polypeptide of Mr 90,000, corresponding to the entire xynZ gene product, was detected in a culture supernatant from C. thermocellum grown on cellulose. By immunological detection, xylanase Z was shown to be associated with a cellulose-binding, high-molecular-weight fraction whose properties coincided with those described previously for the cellulose-degrading complex of C. thermocellum known as the cellulosome.
Full text
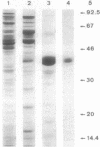
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Setter E., Lamed R. Organization and distribution of the cellulosome in Clostridium thermocellum. J Bacteriol. 1985 Aug;163(2):552–559. doi: 10.1128/jb.163.2.552-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Coughlan M. P., Hon-Nami K., Hon-Nami H., Ljungdahl L. G., Paulin J. J., Rigsby W. E. The cellulolytic enzyme complex of Clostridium thermocellum is very large. Biochem Biophys Res Commun. 1985 Jul 31;130(2):904–909. doi: 10.1016/0006-291x(85)90502-9. [DOI] [PubMed] [Google Scholar]
- Grépinet O., Chebrou M. C., Béguin P. Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum. J Bacteriol. 1988 Oct;170(10):4582–4588. doi: 10.1128/jb.170.10.4582-4588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Sakajoh M., Halliwell G., Madia A., Demain A. L. Saccharification of Complex Cellulosic Substrates by the Cellulase System from Clostridium thermocellum. Appl Environ Microbiol. 1982 May;43(5):1125–1132. doi: 10.1128/aem.43.5.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamed R., Setter E., Bayer E. A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J Bacteriol. 1983 Nov;156(2):828–836. doi: 10.1128/jb.156.2.828-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F., Coughlan M. P., Mori Y., Ljungdahl L. G. Macromolecular Organization of the Cellulolytic Enzyme Complex of Clostridium thermocellum as Revealed by Electron Microscopy. Appl Environ Microbiol. 1987 Dec;53(12):2785–2792. doi: 10.1128/aem.53.12.2785-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre J., Longin R., Millet J. Purification and properties of an endo-beta-1,4-glucanase from Clostridium thermocellum. Biochimie. 1981 Jul;63(7):629–639. doi: 10.1016/s0300-9084(81)80061-2. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Wiegel J., Mothershed C. P., Puls J. Differences in Xylan Degradation by Various Noncellulolytic Thermophilic Anaerobes and Clostridium thermocellum. Appl Environ Microbiol. 1985 Mar;49(3):656–659. doi: 10.1128/aem.49.3.656-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]